

46423-AC3
Development of Catalytic Reactions using Well-Defined Monomeric Cu(I) Complexes with Non-Dative Heteroatomic Ligands: Fundamental Exploration of Scope and Mechanism of C-X (X = N, O or S) Bond Forming Reactions
Monomeric Cu(I) complexes of the type (NHC)Cu(X) (NHC = N-heterocyclic carbene; X = NHPh, OR or SR), which are relatively rare examples of well-defined monomeric Cu systems with amido, thiolate, alkoxide and related non-dative ligands, were used to explore the catalytic formation of C-heteroatom bonds including aryl amination reactions and the addition of N-H and O-H bonds across carbon-carbon multiple bonds. Three manuscripts are in preparation as a result of the funded research in the past year.
(NHC)Cu(NHPh) complexes catalyze aryl amination reactions. Since (NHC)Cu(Me) complexes react with amines to release methane and generate (NHC)Cu(NHR) complexes, either (NHC)Cu(NHPh) or (NHC)Cu(Me) can be used the catalyst precursor. Research efforts were focused on developing the scope of reaction and understanding the mechanism. For the catalytic transformations, the following details have been elucidated:
1) (IMes)Cu systems are more efficient catalysts than (IPr)Cu catalysts {IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene; IMes = 1,3- bis(2,4,6-trimethylphenyl)imidazol-2-ylidene}.
2) Aryl iodides and triflates are reactive, but aryl chlorides, bromides and tosyl reagents do not undergo catalytic transformation.
3) A variety of bases and solvents can be utilized, but under most conditions toluene and K2CO3 optimize yields.
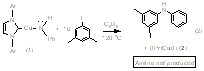
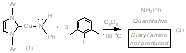
(IPr)Cu(NHPh) (1) reacts with iodobenzene to generate Ph2NH (60% yield), (IPr)Cu(I) (60% yield), aniline (40% yield) and a second uncharacterized Cu product (40% yield) (eq 1). To our knowledge, this is the first example of a well-defined Cu amido complex reacting with aryl halide to generate aryl amine product. Mechanistic studies suggest that the formation of aniline is not likely due to an initial Cu-Namido bond homolysis to give aniline radical. These results suggest that the iodide functionality might be providing electronic activation for the para-CH bond of iodobenzene. To test this possibility, complex 1 was reacted with 1-iodo-3,5-dimethylbenzene, for which the C-H bond para to the iodide functionality is sterically protected by the methyl groups. This stoichiometric reaction cleanly produces (IPr)Cu(I) (2) and the corresponding aryl amine without production of aniline (eq 2). The reaction of complex 1 and 1-iodo-3,5-dimethylbenzene to produce only diaryl amine (and no aniline) suggests that steric protection of the iodo functionality should increase the production of aniline. Indeed, the reaction of (IPr)Cu(NHPh) (1) and three equivalents of 1-iodo-2,6-dimethyl benzene yields nearly quantitative production of aniline (eq 3). In this reaction, a single Cu complex is initially produced, which we presume is (IPr)Cu(C6H2Me2I), but at longer reaction times multiple (IPr)Cu species grow in, precluding isolation and characterization of the putative Cu aryl product that would result from C-H bond cleavage.
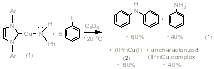
Studies of olefin hydroamination and hydroalkoxylation have been extended to intramolecular variants including reactions with alkynes. (NHC)Cu(Me) complexes catalyze the formation of five- and six-member heterocycles for substrates that possess electron-withdrawing groups. In addition, (IPr)Cu(Me) serves as a catalyst precursor for the regioselective intramolecular addition of O-H groups across alkyne moieties (eq 4). Mechanistic studies indicate that (IPr)Cu(Me) reacts with the alcohol to generate a Cu acetylide complex (Figure 1). Although the Cu-acetylide serves as a catalyst, it is not the resting state of the catalyst. Mechanistic studies and efforts aimed at determining substrate scope for the intramolecular transformations are on-going.
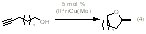
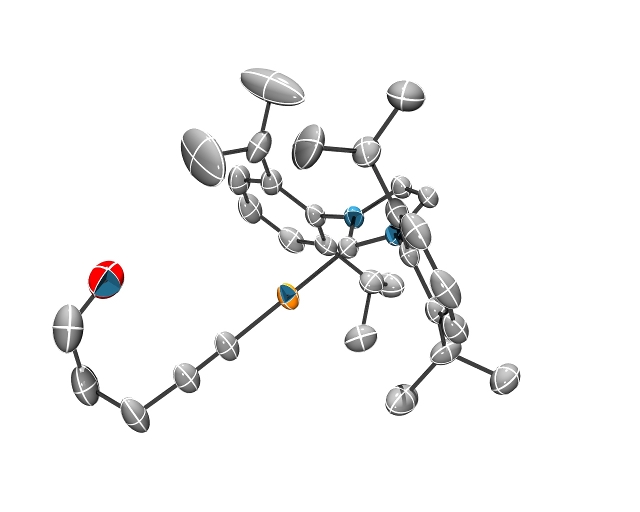
Figure 1. ORTEP of (IPr)Cu{C≡C(CH2)3OH}
Non-dative heteroatomic ligands exhibit diverse reactivity that is heavily influenced by the identity and oxidation state of the metal center as well as the identity of the ancillary ligands. Until recently, examples of non-dative ligands coordinated to late transition metals in low oxidation states were relatively rare. Detailed recent studies have revealed that bonding between late transition metals and non-dative ligands can be relatively strong, yet highly reactive. Despite recent interest in amido complexes with high d-electron counts and new pulse techniques that have rendered 15N NMR spectroscopy more routine, a detailed study of amido systems using 15N NMR spectroscopy has not been reported. 15N NMR chemical shifts have been determined for amido and amine complexes of Ru(II), Pt(IV), W(II) and Cu(I), and in combination with computational chemistry, chemical shifts are being analyzed with respect to metal-nitrogen bonding, especially as a function of d-electron count.