44160-AC4
Investigation of Dye-Protein Interactions and Optimization of Fluorescence-Based Assays for Target Binding of Calmodulin
Many modern methods of sensitive analysis are based on fluorescence properties. These techniques often rely on a fluorescence probe attached to a protein. An important example is fluorescence polarization (FP) assays for binding affinities to proteins. In this project, we are using fluorescence methods to detect binding to calmodulin (CaM), a Ca2+ signaling protein. The goals of this project are (1) to characterize the influence of electrostatic interactions on the interactions of dyes attached to CaM, and (2) to use this knowledge to develop sensitive assays for target binding by CaM. In the initial stages of the project, we have characterized the interactions of several dye molecules having different charges to CaM (see our 2007 PRF report). We are now applying these methods to measure binding affinities of proteins and peptides for CaM in a high-throughput manner.
FP Competition Assay
We have developed a FP competition assay to measure the binding affinities of CaM to multiple targets in a high-throughput setting. The assay is based on competition for binding of CaM to a fluorescently labeled peptide (the tracer) derived from the CaM-binding domain of myosin light-chain kinase. This peptide (M13) was designed to include a cysteine residue at the N-terminus of the 20-amino acid peptide. This cysteine residue was labeled with Atto-465 malemide and purified by size-exclusion chromatography.
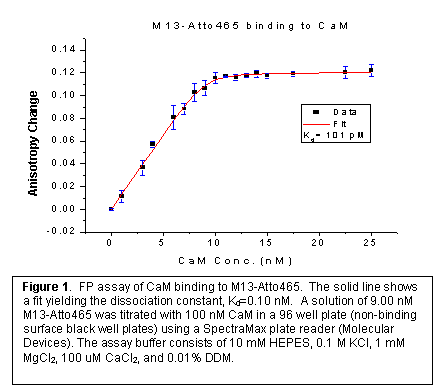
In order to determine the dissociation constants of other CaM binding targets by the competitive replacement of the fluorescently labeled tracer, the dissociation constant Kd of M13-Atto465 with CaM was determined by fluorescence anisotropy measurements (Figure 1). n-Dodecyl-b-D-maltoside (DDM) was used to prevent peptide adsorption onto the surfaces of the microwells. The concentration of the labeled peptide and the CaM was determined by a micro-BCA assay. Although at higher concentration, the concentration of DDM weakly influences the binding of M13-CaM-Atto465 to CaM, a concentration of DDM was chosen low enough so that this affect is negligible, but high enough to hinder adsorption of proteins to the microwell surfaces.
Single-molecule FP measurements.
Another application of fluorescence detection is
single-molecule measurements. Prof.
David Arnett of Northwestern College (Orange City, IA) recently carried out
single-molecule fluorescence-polarization measurements under a PRF summer
supplement. Prof. Arnett developed a
four-channel single-molecule system to carry out simultaneous measurement of single-molecule
F
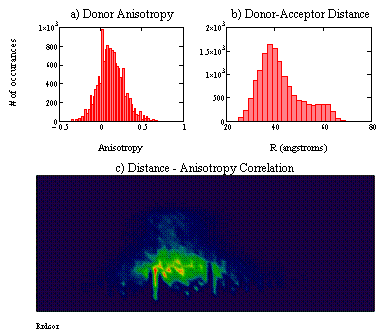
Figure 2: Single-molecule FRET results for CaM labeled with AF488 (donor) and Texas Red (acceptor). Energy transfer and anisotropy were measured following excitation with a picosecond pulsed laser operating at 482 nm. Panel (a) shows a histogram of the observed donor anisotropy. Panel (b) shows the corresponding histogram of donor-acceptor distances calculated from FRET efficiencies. Panel (c) shows the correlation between donor anisotropy (x-axis) and the observed distance (y-axis). The color map represents the number of molecules with a given combination of donor anisotropy and distance. The axis scales are equivalent to those in panels (a) and (b).