

46760-AC7
Nanostructuring and Thermal Behavior of Polymers: Toward Improving Longevity of Important Petroleum Derived Products
Nanostructuring and thermal behavior of polymers: Toward
improving longevity …
(46760-AC7 + SUMR 46760.01-AC7)
The original proposal was intended for 2 years, of which most of the 1st year was planed to devote to synthesis of PS-clay materials of different grafting density. Since the funding was provided for 1 year only, the efforts planned had to be reorganized. The project had to be started on January 1st 2008 because that was earliest availability date of Dr. Kai Chen, whose hiring was crucial to make the 1 year research effort successful. Therefore, the present report covers the results 8 months work (1/1/08 – 8/31/08)
As a part of the overall project effort to
understand the effect of nanostructuring on the stability of polymers, we have
also focused on the process of sol-gel and gel-sol transition in PS-clay
materials. The endeavor has in part become possible thanks to the additional
funding (46760.01-AC7) under the SUMR program. Our efforts have focused on the
application of differential scanning calorimetry (DSC) to study the sol-gel and
gel-sol transition in virgin polystyrene (PS) and PS-clay materials dissolved
in carbon disulfide (CS2). The two PS-clay nanocomposites studied
have respectively been of exfoliated brush and intercalated structures.
Compared to virgin PS, the behavior of the brush material has shown little
difference, whereas the intercalated material has demonstrated a markedly
larger heat of both sol-gel conversion (gel formation) and gel-sol conversion
(gel melting). Isoconversional kinetic analysis of DSC measurements has
revealed substantial differences in the melting behavior of the gels prepared
under isothermal and continuous cooling conditions. For isothermally prepared
gels, the effective activation energy is independent of the extent of
conversion that suggests the process is dominated by a single pathway gel-sol
conversion. The gels prepared under continuous cooling conditions have
demonstrated a significant increase in the effective activation energy with
increasing the extent of conversion. It is suggested that the melting of the
nonisothermally prepared gels occurs via competition of the gel-sol conversion
and the formation of new gel structures. To our knowledge, this is the first
study of the effect of nanostructuring on the dynamics of sol-gel and gel-sol
transformation. The results have been presented in a paper that has just been
accepted for publication by Macromolecular
Chemistry and Physics, which will feature our paper on the cover of the
issue N23! The SUMR scholar, Ashley Baker played an important role in this
study working in close collaboration with Dr. Kai Chen.
[1] Weimer, M.W.; Chen, H.;
Giannelis, E.P.; Sogah, D.Y. J. Am. Chem.
Soc. 1999, 121, 1615. [2] Puts, R.D.; Sogah, D.Y. Macromolecules 1997, 30, 7050. [3] Dao, J.; Benoit, D.;
Hawker, C.J. J. Polym. Sci. Part A:
Polym. Chem. 1998, 36, 2161.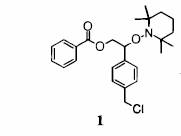 The
major step in preparing the proposed PS-clay materials is synthesis of
alkoxyamine initiator for Nitroxide-mediated polymerization (NMP). Our
synthetic work was originally based on the literature reports.[1], [2] Substantial amount amount of time and effort have
been invested to synthesize Benzoic acid
2-(4-(Chloromethyl)phenyl)-2-(2,2,6,6-tetramethylpiperdin-1-yloxy)ethyl Ester
(1) by mixing benzoyl peroxide, TEMPO (2,2,6,6-tetramethylpiperidin-1-yloxyl),
and 4-vinylbenzyl chloride and heating the mixture for 24 hours at 80 °C as
suggested in the literature.1
The
major step in preparing the proposed PS-clay materials is synthesis of
alkoxyamine initiator for Nitroxide-mediated polymerization (NMP). Our
synthetic work was originally based on the literature reports.[1], [2] Substantial amount amount of time and effort have
been invested to synthesize Benzoic acid
2-(4-(Chloromethyl)phenyl)-2-(2,2,6,6-tetramethylpiperdin-1-yloxy)ethyl Ester
(1) by mixing benzoyl peroxide, TEMPO (2,2,6,6-tetramethylpiperidin-1-yloxyl),
and 4-vinylbenzyl chloride and heating the mixture for 24 hours at 80 °C as
suggested in the literature.1