45076-G6
Electron Transfer in Cluster Anions: Dissociative Attachment Processes in Real Time
Our long term goal is to develop an experimental methodology to probe electron transfer initiated chemical dynamics on the timescale of molecular motion. The excess electron in a cluster anion is often localized on a particular moiety (surrounding molecules retain largely neutral character). Initiation should be possible with an ultrafast laser pulse effecting intracluster electron transfer. The results of studies over the past year suggest promise for such a strategy and provide valuable insights into photodetachment processes in cluster and molecular anions. At this stage we have two experimental foci, to understand the relationship between the photoelectron angular distributions (PADs) and the detachment process and to find suitable systems for electron transfer studies.
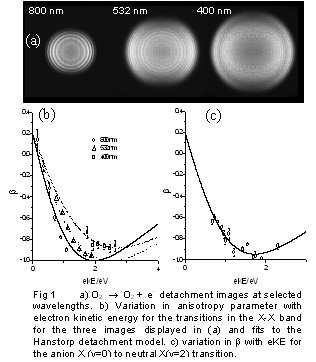
PADs are often stated to represent “a signature of the parent orbital.” However, as our cluster anion results show, we should append “for direct photodetachment.” Furthermore, consideration of only the parent orbital is insufficient. Our photodetachment experiments clearly reveal the influence of the detachment channel on the PAD. The PAD is characterized by the anisotropy parameter, β which for anion detachment depends on the electron kinetic energy (eKE). Until recently, studies of the variation in β with eKE have not been straightforward. We use imaging detection and a broadly tunable, linearly polarized laser to systematically study eKE induced changes in β for O2-. The superoxide photoelectron spectrum1 has several well resolved vibronic bands and the HOMO of the superoxide anion is similar to that of an atomic d orbital, allowing the use of a relatively simple atomic detachment models to predict β.2
An image (single detachment wavelength) provides β values for each vibronic transition. Using a solely parent orbital based model yields a fit to the β values from an image. This should predict the behavior at any other detachment wavelength. The experimental observations differ from this expectation. In fact a different curve is required for each photodetachment wavelength. However, if the data are grouped according to neutral vibrational excitation, each vibronic transition requires a different curve but this predicts β over the whole eKE range for a given vibrational level. In addition to the parent orbital, the PAD is dependent on the vibrational channel which can be rationalized in a Franck-Condon like picture. The overlap of the detachment orbital and the continuum wavefunctions is related to the vibrational level of O2.
Our cluster anion based experiments reveal the presence of intra-cluster electron-molecule interactions during the detachment process. These studies of the PAD associated with cluster anion detachment yield insights into intracluster photoelectron interactions. Previous work has shown that cluster anions such as I-·Ar, I-·CH3CN and I-·H2O have virtually indentical PADs to those of free I- detachment at similar eKE,3 these detachment processes are direct. Electrons detached from free I- with 0<eKE<3.5 eV yield a negative β value which corresponds to a distribution polarized perpendicular to the laser polarization axis (although β rises to 0 as the threshold is approached). Photoexcitation of I-·CH3I and I-·CH2I2 shows completely different results. The distribution peaks parallel to the laser polarization axis near threshold and varies in a manner that is totally different from that of the I-·X clusters above. The low kinetic energy electrons encounter a scattering resonance leading to the alteration of the PAD. Thus, the ns experiment probes the nature of the free electron-molecule interaction.
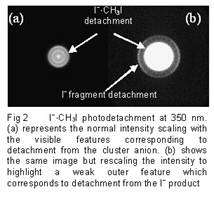
The ns detachment experiments on I-·CH3I also reveal the presence of a fragmentation channel competing with detachment. These latter results are very exciting, allowing characterization of the dissociation channel prior to fs pump-probe studies of fragmentation (ongoing work). For a narrow band of photon energies around the detachment threshold, the experiments show the presence of free I-. In this region, excitation creates a transient excited molecular anion via electron attachment through a vibrational Feshbach resonance.4 The transient anion then decays, either losing the electron through autodetachement (seen in the images as a central spot) or by fragmentation into I-. The ns laser allows us to detect the fragment with the same pulse that causes the excitation but of course doesn't allow time resolved measurements. Interestingly in the case of I-·CH2I2, the dissociative channel is also observed but lies a little higher above the cluster detachment threshold. The identification of the energetics of the intracluster fragmentation channels have paved the way for fs time resolved dissociative attachment studies that are ongoing in our laboratory at the present time.
1 Ervin, K.M. ; Anusiewicz, I. ;Skurski, P.; Simons, J.;Lineberger,
W.C. J.Phys.Chem.A, 107, 8521 (2003). 2