

44510-AC5
Surface Reactivity of Radicals and Ions During Plasma-Catalytic Removal of Nitrogen Oxide (NOx) Pollutants
This project's focus is elucidation
of fundamental chemical processes occurring at surfaces during plasma-catalytic
removal of nitrogen oxides (NOx). Mechanisms for NO removal were investigated
using the imaging of radicals interacting with surfaces (IRIS) technique, which
examines steady-state reactivity of plasma-generated species at catalytic
surfaces. The second funding cycle
focused on fundamental gas phase density studies using optical emission
spectroscopy (OES) and laser-induced fluorescence (LIF, Figure 1) in the
presence of catalysts. We also expanded
the project to other areas of plasma pollution control, namely detection of
organic contaminants in water streams.
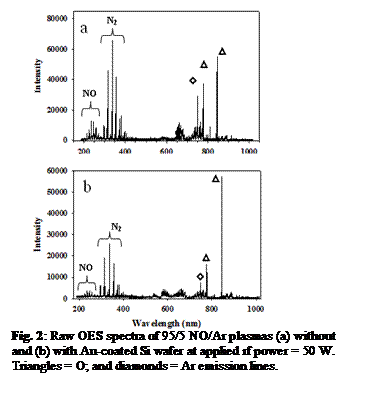
OES Studies. Experiments here focused on using three-way
catalytic converter surfaces of platinum and gold. Notably, Au-coated Si wafers were most efficient
in removing NO from plasmas (either NO, NO/Ar, or N2/O2/Ar). The Figure 2 OES
spectra show Au (Fig. 2b) significantly decreases the NO signal at the lowest applied
rf power (P), 50 W. At P
> 50 W, NO is effectively eliminated.
Over time, the Au loses its ability to remove NO from the plasma. Current data collection efforts focus on understanding
the nature of Au surfaces and how they interact with the gases.
NOx are formed when fuel is burned at high temperatures, and can be produced through gas-phase reactions. We have observed this in N2/O2 plasmas with and without additives that simulate the environment in exhaust fumes. Our results demonstrate the amount of H2O vapor added does change observed trends. The additional source of oxygen results in competing reactions that occur primarily at elevated P. At high P, the NO signal decreases due to increased dissociation of the NO formed by reaction of N + O.
Rotational Temperatures. Knowledge of energy partitioning is important to an overall understanding of the chemistry occurring in our plasmas. We characterized the rotational temperature (ΘR) of NO in our plasmas, Table 1. These data were collected utilizing LIF spectroscopy. For 100% NO and 90/10 N2/O2 plasmas, ΘR does not change appreciably with P. Conversely, ΘR in the NO/Ar mixture appears to increase slightly with P, although it is within the experimental error. Moreover, ΘR in the NO system is somewhat elevated relative to values found in NO/Ar and N2/O2 mixtures. Thus, NO is rotationally cooled by collisions with Ar or when formed via bimolecular collisions in N2/O2.
Table 1. Rotational Temperatures for NO in Different Plasmas (K)
| |||
Applied rf power (W)
| NO (100%)
| NO/Ar (12:88)
| N2/O2 (90/10)
|
25 | 356 ± 12 | 317 ± 10 | 317 ± 36
|
50 | 342 ± 12 | 312 ± 9 | 323 ± 40 |
75 | 366 ± 15 | 321 ± 32 | 322 ± 15 |
100 | 366 ± 20 | 312 ± 13 | 325 ± 18 |
125 | 368 ± 34 | 339 ± 25 | 320 ± 13 |
150 | 366 ± 30 | 348 ± 24 | 320 ± 12 |
Organic Contaminant Detection and Removal. We expanded our studies to plasma pollution control, including detection and removal of organic contaminants from water sources. Although large volumes of water may not be treatable with plasma methods, the ability to detect contaminants in ultrapure water (e.g those used in the microelectronics industry) was also a goal. Among the potential contaminants, those associated with fuel oxygenate additives (methyl tert-butyl ether (MTBE)) are of concern as MTBE partitions into aqueous phases. We used OES in our plasmas for detection of methanol and MTBE in water samples. Using CO* emission, a detection limit of 0.01 ppm was determined for each organic contaminant. Complementary mass spectrometry data were collected to explore decomposition mechanisms for both CH3OH and MTBE. We found that CH3OH decomposition is achieved primarily via an oxidative dehydrogenation mechanism, whereas MTBE abatement occurs via both decomposition and oxidation. This work has resulted in one submitted manuscript.
Summary/Impact. In the second year of this project we have further explored plasma-catalytic processes involving NOx removal, including gas-phase characterization of NO. We have expanded the scope to include other projects that have an environmental focus. Michelle Morgan is the graduate student working on the NOx aspects of the project. Kristina Trevino was responsible for the MTBE and methanol water remediation project. Both students have presented their work at three different conferences this year (National AVS, Regional ACS, GRC on Plasma Science). In addition, a new graduate student who joined the group in the spring of 2008 will be working on analysis of other pollutants such as SOx and NO2. We are continuing to build on our results via development of additional environmentally-related projects, including modification of materials used in aqueous separations (e.g. desalination).