47077-AC10
Bioinspired Hybrid Systems for the Photoelectrocatalytic Generation of Solar Hydrogen and Fuels
The ultimate goal of this project is to generate highly efficient and long-term stable photo-electro catalytic cells for the generation of either hydrogen or the combined production of hydrogen and methane. My research aims at the design of photoelectrochemical cells that reduce water and carbon dioxide at a photocathode and oxidize water at a photoanode.
Five of my graduate students have been working towards this goal during the last year.
Dr. Xiaoxuan Leaym has graduated from Kansas State University and went to a job in industry. At NanoScale she is concerned with organic chemistry and photochemistry on inorganic nanoparticles.
Pubudu Gamage, who will finish his Ph.D. in the summer of 2009, constructed the photochemical micro-reactor for the sacrificial photolyses. He has successfully completed the sacrificial hydrogen evolution stage of this project. His research in 2008/2009 will be devoted to a photoelectrochemical system that works without sacrificial donors. Pubudu plans to pursue his chemical career in the petroleum industry.
Tej Shrestha's research is concerned with the development of efficient catalysts for the generation of methane and other small hydrocarbons from carbon dioxide. Tej plans to return to Nepal after the completion of his Ph.D. The development of decentralized photochemical methods for harvesting solar power is of great importance to him.
Thilani Samarakoon works on composite photocathodes for the reduction of water and carbon dioxide. She would like to pursue her career in academics.
Matthew Basel has performed Atomic Force Microscopy experiments, which helped to characterize the Ru(0)-nanoparticles and the titanium dioxide surfaces. He would like to pursue his career as a postdoctoral fellow at an international research institute.
This ACS-PRF grant helped me to restart my alternative energy research after I moved from the University of Karlsruhe/Germany to Kansas State University. Since last year, I have become a member of the "Kansas Energy Consortium", which successfully won our state's support for an NSF-EPSCoR grant. We are in the process of applying for a DOE-center grant as well.
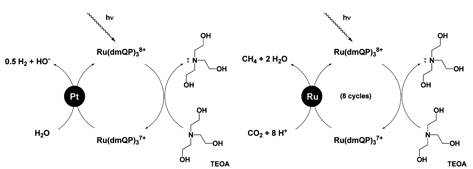
Today it is apparent that a rapid development of alternative sources of energy is needed to satisfy the world's demand for an adequate energy supply. Ruthenium-(II)quaterpyridinium complexes exhibit superior reactivity as sensitizer-relay-assemblies (SRAs) in sacrificial systems for water and hydrocarbon reductions, while harvesting most of the visible fraction of the incident solar spectrum including the ultraviolet rays. A typical reaction scheme using triethanolamine (TEAO) as sacrificial donor is shown here.
Sacrificial
reaction schemes for water and carbon dioxide reduction Phosphonate-tethered
Ru(II)quaterpyridinium complexes were synthesized from
Ru(II)-tris-quaterpyridyl. As these complexes form stable adhesive layers on
ITO (indium tin oxide), a series of differential pulse voltammetry experiments
were carried out to measure their ground state and excited state redox
potentials. These reductive potentials were compared with the reductive
potentials of carbon dioxide to methane and water to dihydrogen reactions. Our
experiments confirmed that it is thermodynamically possible to oxidize water
and to reduce carbon dioxide by using phosphonate-tethered
Ru(II)quaterpyridinium complexes. Especially the eight-electron reduction from
carbon dioxide to methane requires only a small thermodynamic driving force (E
= -0.25V vs. SHE) and is, contrary to one electron reduction of carbon dioxide
(E > -2.0V vs. SHE), relatively easy to achieve! A series of ruthenium(0)-
and platinum(0) catalysts on titanium dioxide (Degussa P25) were prepared and
the concentration and size-distributions of the catalytic
ruthenium(0)-nanoparticles optimized. Nanoparticles of approximately 20-50nm in
diameter showed maximal catalytic activities, the optimum pH for the reduction
of carbon dioxide was 7.25.
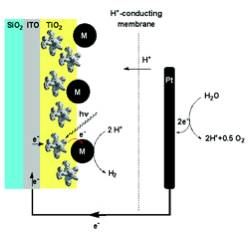
Photoelectrochemical
reactor for hydrogen evolution: M=platinum nanoparticle Summary:
Our research is proceeding as planned.