
Back to Table of Contents
47142-GB1
Pd(0)-Catalyzed [3+2] Cycloadditions Involving Novel Epoxide and Aziridine Containing Trimethylenemethane Precursors
Kevin L. Jantzi, Valparaiso University
During
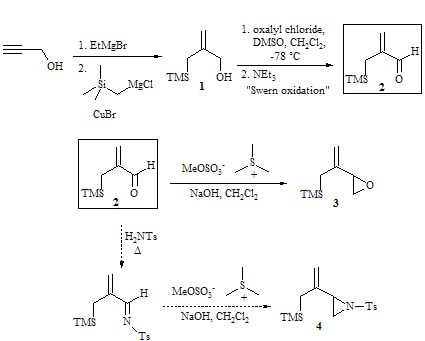
the last year, four students and I have worked on this project. We have synthesized a large amount of
compound 1, an important
intermediate in the synthesis of our desired epoxide (3) and aziridine (4)
precursors for the Pd(0)-catalyzed Trimethylenemethane (TMM) cycloaddition
reactions. We have also scaled up the
Swern oxidation and can routinely do the reaction on a 10 mmol scale with good
results and yields. We have also made
some initial studies related to the formation of compound 4 which indicate that it may be possible to use intermediate
aldehyde 2 as a precursor to both
compounds 3 and 4.
Based
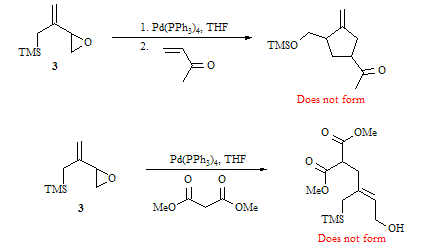
on a large number of reactions using various conditions, it is quite clear that
compound 3 does not undergo the
Pd-catalyzed reactions shown below.
Apparently, the epoxide does not open to form the intermediate π-allyl
palladium complex, even though similar model systems by Trost have been shown
to undergo epoxide opening. We have been
able to repeat these two reactions using known precursors from the literature,
so it is clear that our technique and reagents are fine. It may be that the large TMS group of
compound 3 is blocking the approach
of the palladium catalyst. In any event,
we plan to use a variety of Lewis acids (e.g., TMSOAc, BF3·OEt2,
TMSCl) to promote opening of the epoxide. We also
plan to use a more electron rich palladium catalyst which may better promote
ring opening.
Back to top




