

46098-AC6
Femtosecond Infrared Spectroscopy of Proton-Coupled Electron Transfer Dynamics
Ultrafast Proton Dynamics During Proton-Coupled-Electron-Transfer and Excited-State-Proton-Transfer
Abstract
Asymmetric doubly hydrogen bonded interfaces between amidine and carboxylic acid functionalities are important structural motifs for biological systems exhibiting proton transfer. Such asymmetric proton interfaces are thought to be important for the photo-stability of DNA and in biological energy-transfer systems involving proton-coupled-electron-transfer (PCET). A limiting case of PCET is excited state proton transfer (ESPT). ESPT occurs from a number of polyaromatic amidines, where the proton transfer is mediated by the surrounding hydrogen-bonding network either through a dimer counterpart or solvent molecules. Excited state dynamics of such systems have been extensively studied in the gas phase and solution through ultrafast visible spectroscopy in an effort to determine if the proton transfer occurs via a stepwise or concerted mechanism [1-4]. However, this is still a controversial area of research [3,4]. In such systems the hydrogen bonded stretching vibrations of the NH or OH bond couple directly into the proton transfer coordinate. We apply ultrafast vibrational spectroscopy to directly monitor the proton transfer mechanism through the interchange of hydrogen-bonding donor and acceptor. The present study is focused two symmetric and two asymmetric cyclic doubly hydrogen-bonded dimers. The four dimers are the two homo-dimers of 7-azaindole (7-AI) and 1H-pyrrolo[3,2-h]quinoline (PQ) and their hetero-dimer counterparts with acetic acid.
Current Status
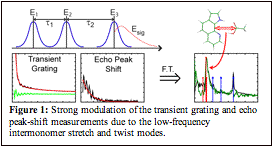 We have performed a complete set of third order
spectroscopies: transient grating, echo peak shift, pump probe and full 2D-IR
surface of all four dimers in the NH region. This has lead to a complete understanding
of the NH vibrational dynamics in the electronic ground state. Whereas the
linear spectra might be dominated by Fermi-resonances, the transient grating
and echo peak-shift measurements have shown that the vibrational dynamics are
dominated by the low-frequency inter-monomer stretching and twisting
vibrations. These are the low-frequency modes that directly modulate the
hydrogen-bond strength of the NH bond. The results are accepted for publication
in J. Phys. Chem. B [5]. This finding is backed up by DFT calculations that
characterize the effect of the low-frequency inter-monomer stretching vibration
on the high-frequency NH and OH modes. Frequency dispersed pump-probe
experiments and 2D surfaces fully resolve the intricate beating pattern observed
in the transient grating and echo peak-shift measurements and is completely
explained by the hydrogen-bonding strength of the vibration. Energy is observed
to flow from the NH modes into the Fermi-resonances and strong coherent beating
is observed between the different cross-peaks. A manuscript based on the
pump-probe and 2D-IR experiments along with the DFT calculations is in
preparation.
We have performed a complete set of third order
spectroscopies: transient grating, echo peak shift, pump probe and full 2D-IR
surface of all four dimers in the NH region. This has lead to a complete understanding
of the NH vibrational dynamics in the electronic ground state. Whereas the
linear spectra might be dominated by Fermi-resonances, the transient grating
and echo peak-shift measurements have shown that the vibrational dynamics are
dominated by the low-frequency inter-monomer stretching and twisting
vibrations. These are the low-frequency modes that directly modulate the
hydrogen-bond strength of the NH bond. The results are accepted for publication
in J. Phys. Chem. B [5]. This finding is backed up by DFT calculations that
characterize the effect of the low-frequency inter-monomer stretching vibration
on the high-frequency NH and OH modes. Frequency dispersed pump-probe
experiments and 2D surfaces fully resolve the intricate beating pattern observed
in the transient grating and echo peak-shift measurements and is completely
explained by the hydrogen-bonding strength of the vibration. Energy is observed
to flow from the NH modes into the Fermi-resonances and strong coherent beating
is observed between the different cross-peaks. A manuscript based on the
pump-probe and 2D-IR experiments along with the DFT calculations is in
preparation.
Future Direction
Over the course of the next year we plan to model and report the complete characterization of the NH vibrational dynamics in the electronic ground state. Furthermore, we plan to greatly extend the UV photo initiated studies directly monitoring the proton motion during the excited state proton transfer reaction in the hope to discern a concerted vs. stepwise proton transfer mechanism.
[1] J. Waluk, "Hydrogen-Bonding-Induced Phenomena in Bifunctional Heteroazaaromatics," Acc. Chem. Res. 41, 832 (2003).
[2] A. Kyrychenko, and J. Waluk, " Excited-State Proton Transfer through Water Bridges and Structure of Hydrogen-Bonded Complexes in 1H-Pyrrolo[3,2-h]quinoline: Adiabatic Time-Dependent Density Functional Theory Study," J. Phys. Chem. A 110, 11958 (2006).
[3] S.Takeuchi, and T. Tahara, "The answer to concerted versus step-wise controversy for the double proton transfer mechanism of 7-azaindole dimer in solution," Proc. Natl. Acad. Sci. 104, 5285 (2007).
[4] O.-H. Kwon, and A. H. Zewail, "Double proton transfer dynamics of model DNA base pairs in the condensed phase," Proc. Natl. Acad. Sci. 104, 8703 (2007).
[5] P.B. Petersen, S.T. Roberts, K. Ramasesha, D.G. Nocera, A. Tokmakoff, "Ultrafast N-H vibrational dynamics of cyclic doubly hydrogen-bonded homo- and heterodimers," J. Phys. Chem. B, accepted.