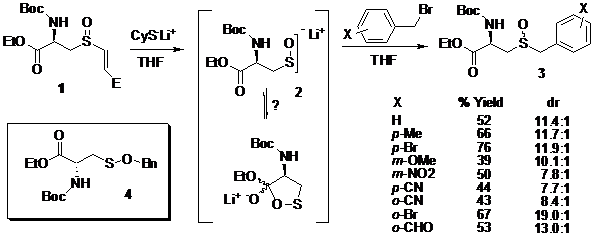45984-AC1
New Low Valent Sulfur and Selenium Acid Derivatives: Reactivity and Synthetic Applications
Sulfenic acid anions have are slowly being recognized as valuable entities for the constructions of sulfur containing organic compounds. Recent work by Madec1 clearly shows their value for the formation of enantioenriched aryl sulfoxides. Any synthetic protocol for their synthesis must work within the limitations of their stability. Specifically, sulfenates are generally not isolable, but must be created from the appropriate precursor and then reacted in the same vessel. One of the viable methods for sulfenates involves their release from b-sulfinyl acrylates. In our group, this is generally achieved by treating the b-sulfinyl acrylates with cyclohexanethiolate as a nucleophile.2 The thiolate initiates an addition-elimination reaction, releasing sulfenate anion for further chemistry. The b-sulfinyl acrylates are generally prepared from a conjugate addition/oxidation sequence. However we have developed a protocol for selected E-b-sulfinyl acrylates by utilizing a new reagent, cesium 2-Z-carbomethoxyethenethiolate. A manuscript is nearly ready for submission and PRF funding was a partial but important contributor to this paper.
This strategy is most applicable for targeted sulfenates that are base sensitive. As such we have been investigating the chemistry of a protected cysteinesulfenate derivative. Our addition elimination chemistry proceeds with minimal perturbation of the sensitive a-proton that normally makes amino acid chemistry challenging.
Under this plan, cysteine derivative 1 was required for cysteinesulfenate liberation. During the first period of
PRF funding, two different syntheses of 1 were fully evaluated. Analogs
of 1 have previously been prepared by
The sulfenate (2) was then generated via thiolate treatment and captured with a selection of electrophiles including several substituted benzyl bromides affording diastereomeric sulfoxides (3, Scheme 1). All possible products were prepared by conventional means and these compounds were used as identification and calibration standards. Chiral HPLC analysis was used to establish the diastereomeric ratio. Yields range from 38-76% and diastereomeric ratios range from 7.7:1 to 19.0:1. In the burgeoning field of sulfenate chemistry these diastereoselectivities represent a significant achievement.
An interesting feature of the cysteine sulfenate chemistry is the rate of the alkylation chemistry. Compared to other sulfenate alkylations, this sulfenate does not readily alkylate with benzyl bromide. Indeed, sulfoxide formation is significantly accelerated by exposure of the mixture of water and/or silica gel. It is thought that the particular sulfenate does not behave like others and perhaps its alkylation chemistry is retarded for some reason. The possibility of O-alkylation has been ruled out, by independent synthesis of sulfenate ester 4. To account for the slow alkylation, it is suggested that the sulfenate reversibly closes onto the proximal ester. NMR analysis of the concentrated crude reaction mixture indicates the absence of the ester carbonyl. Although the structural assignment of the intermediate sulfenate is presently tentative, this nevertheless represented a new mode of sulfenate reactivity and investigations in this area are continuing.
Scheme 1
It has been proposed that some benzylic sulfoxides diastereoselectively formed by this sulfenate chemistry are
good candidates for intramolecular cyclization chemistry targeting 7 or 8 membered sulfur/nitrogen heterocycles. A number of
reactions of 3 (X = o-Br) have been evaluated, but the
substrate decomposes under the thermal activation required for the cyclization.
Acid mediated removal of the Boc group was achieved
without perturbation of the sulfenyl group, but cyclization attempts of those
substrates did not proceed. Some literature reports suggest different nitrogen
protection may facilitate cyclization, so others are under investigation. Cysteinyl sulfoxide 3 (X = o-CHO) was
prepared to explore cyclocondensation of the amino
acid nitrogen to the aldehyde.
Marcus Verdu,
an MSc candidate since Jan. 2007 and Dr. Suneel P.
Singh, a pdf since late March 2008, have carried this work out. Marcus will
finish his studies in 4-6 months and is soon expected to receive a job offer
from international mining giant Xstrata. Marcus' instrumental skills (NMR,
HPLC, ReactIR) and his
project management skills were viewed very favourably by Xstrata HR personnel. Suneel Singh will be continuing the chemistry into the
second funding period and has been joined by MSc candidate Stefan Soderman whose has received a graduate scholarship through
the Canadian funding agency NSERC.
References Cited 1. Maitro, G.; Vogel, S.; Sadaoui,
M.; Prestat, G.; Madec, D.;
Poli, G. Org. Lett. 2007, 9, 5493. 2. O'Donnell, J.S.; Schwan, A.L. Tetrahedron Lett. 2003, 44,
6293. 3. Aversa, M.C.; Barattucci, A.; Bonaccorsi, P.; Giannetto, P. J. Org. Chem. 2005, 70,1986.