43890-B5
Gemini Surfactant Clay Intercalates for Triphase Catalysis
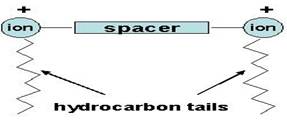
We are pursuing triphase catalysis (TC) which is a unique type of phase transfer catalysis (PTC) using clay as solid phase and support for the catalyst. We have used hectorite clay from the smectite clay family (layered swelling clay) to support the catalysts through intercalation chemistry. We have developed various novel clay supported catalysts in our research using Gemini surfactants. Gemini surfactants essentially consist of two surfactants attached by a spacer group close to the two head groups.
Fig. 1. Schematic
representation of a Gemini surfactant. Gemini surfactants have the technological advantages of
lower critical micelle concentration (CMC), higher surface tension and superior
wetting properties than achievable with traditional monomeric
surfactants. We have synthesized and
characterized Gemini dicationic compounds C16-C2-C16,
C16-C4-C16, C16-C6-C16,
C12-C4-C12, C12-C6-C12
for application in triphase catalytic reactions using
toluene as organic phase and salt water as aqueous phase. The nucleophilic
displacement reaction is given below:
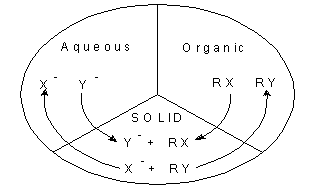
Fig. 2. A triphase catalytic reaction. The reactants from organic and aqueous phase
come to the surface of the solid phase (Gemini intercalated clay) and
react.
While our main goal is to study the effect of Gemini-hectorite clay complex on the kinetics of the displacement
reaction, we are also interested in a clear understanding of the factors that
determine the often very strong selectivity of the clay for certain ions and
the propensity of the hectorite clay to intercalate
Gemini surfactants. For this reason, we
have used X-ray powder diffraction to examine the intercalation of Gemini
surfactants with different space and tail length, focusing particularly on the
space occupied by non-charge-neutralizing moieties. The important difference from the traditional
quaternary ammonium surfactant with one cation is
that the Gemini surfactants carry a double charge and also provide the prospect
of testing the effect of surfactant charge on the intercalation reaction.
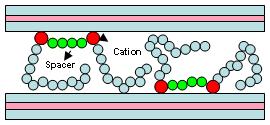
Fig. 3. Intercalated Gemini surfactants in the interlamellar space of hectorite clay. Gemini-clay complexes are also interesting in regards to their
swelling in the presence of solvents such as toluene and aqueous systems. The swelling properties of various
Gemini-clay intercalates in the presence of a single solvent system has been
studied by different groups. Based on
some of our preliminary results (X-ray diffraction as well as reaction
kinetics), we tend to believe that some of the Gemini surfactants have a
greater affinity for adsorbing solvents and swelling. This may help us to be able to tailor clay-Gemini
catalysts with more superior catalytic behavior in triphase
catalytic reactions.
By studying X-ray diffraction patterns and measured basal
spacing we are seeing some differences which must be related to length of the
spacer or tail group of the Gemini surfactants intercalated in the interlamellar spacing of the clay. In our preliminary work regarding triphase catalytic reactions, we have seen some differences
in the rate of the displacement reaction.
However, we have no clear understanding or solid justification for the
differences being observed to date. In
this respect, we decided to repeat some of our work to see if we can verify and
confirm our earlier results. Gemini
synthesis, characterization, as well as clay purification and clay
intercalation reactions take a vast amount of time; however we felt it
necessary to take that route.
I
feel that the impact of this interdisciplinary area of research at SIUE is
significant. In particular, this area of
research has been a great source of experience for my undergraduate students. Neha Parikh and Danielle Reed have worked on
the Gemini synthesis, clay purification and intercalation chemistry. They have also done some preliminary work
towards the kinetics of the displacement reaction. Danielle presented a poster at the 2007 ACS
Midwest Regional Meeting, Kansas City, MO, November 7 – 10, and both Danielle
and Neha each presented a poster at the 235th ACS National meeting, New Orleans, LA April 6-10,
2008. The impact of this project on my
research and my career has also been profound as I have since been tenured and
promoted to Associate Professor. Currently, we are preparing for more experiments to
determine the rate of the nucleophilic displacement
reaction using various Gemini-clay catalysts that we have developed in our laboratory
and are also planning to investigate the effect of stirring rate on the triphase displacement reaction.