45505-GB3
Development of Transition Metal Catalysts for Environmentally Benign Oxidation of Organic Compounds by Nitrous Oxide
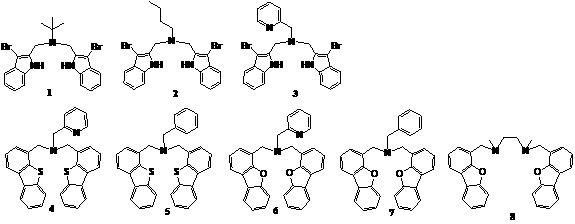
The goal of this project is to develop metal complexes that function as catalyst for environmentally friendly oxidation reactions. The research focus of this year was to find ideal metal complexation conditions for several new multidentate ligands that we had developed in the previous year (1-7) and one new ligand that we synthesized this year (8).
With
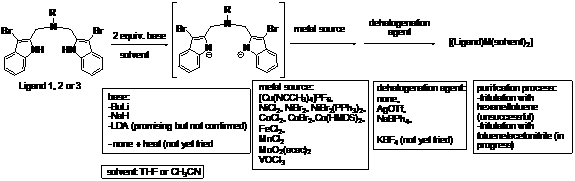
ligands 1, 2 and 3, the metal complexation stage turned out to be a challenge. We have
been trying many combinations of variables for the reaction conditions: metal
sources, solvents, bases, supporting reagents for dehalogenation,
order of addition, and purification process. The variables for ligands 1, 2
and 3 are summarized in the
following scheme.
After
each reaction, the putative product was examined by 1H NMR. In most
cases we saw a mixture of multiple species. In some cases the product could not
be observed by the NMR technique: they were probably paramagnetic. So far, the
conditions that gave relatively clean product (major single product plus some
minor byproducts) was the use of LDA as a base and then mixing it with NiBr2
pre-treated with AgOTf. We are currently refining
this approach, adjusting the reaction time, temperature, solvent, and workup
procedures. We will also test a no-base conditions (direct complexation
between the protonated ligand and metal halide, upon heating).
The
crystallization attempts of some relatively clean products, using
non-coordinating solvents, have apparently resulted in irreversible formation
of less soluble species: probably aggregate of complexes as a result of
bridging of halides over multiple metal centers. We are currently exploring
crystallization conditions always in the presence of a coordinating solvent.
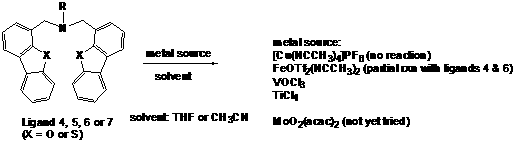
Ligands
4, 5, 6 and 7 all reacted with early transition
metal sources (TiCl4 and VOCl3) and the formation of a
new, clean product was confirmed 1H NMR. However, our efforts to
crystallize the products so far have been unsuccessful. We plan to further
explore different crystallization conditions. An interesting observation was
that while the tridentate ligands 5
and 7 did not react with Fe(OTf)2(NCCH3)2, the
analogous tetradentate ligands 4 and
6 did. The latter, however, showed a
mixture of two species. The reaction conditions should be refined to obtain a
single product.
Ligand
8, which we newly synthesized this
year, showed similar reactivity to that of the non-linear analog 6. We recently found that MoO2(acac)2, pre-treated with 2 equivalents of TfOH, gives a clean product with ligand 8. VOCl3 was another
promising metal source if it was post-treated with AgOTf.
These two reactions are currently repeated in a larger scale so the products
can be fully characterized.
Future
work will involve the full characterization of the metal complexes and testing them
as catalysts for oxidation of hydrocarbons by environmentally benign oxidants
such as N2O and H2O2. Other O-atom transfer
agents such as peroxides, iodosobenzene and hypochlorites will be studied as well.
The
grant paid for chemicals, supplies, and elemental analysis. Four undergraduate
students were actively engaged in this research throughout the year. Two of
them continued in the summer of 2008 and the grant financially supported them
as well as the PI. The tentative results of this project were presented in
three reginal/national meetings: the Southeastern
Regional Meeting of the American Chemical Society 2007 (Greenville, SC, October
24-27, 2007), American Chemical Society National Meeting (New Orleans, 6-10
April 2008), and at the National Conference on Undergraduate Research (Salisbury,
MD, April 10-12, 2008).