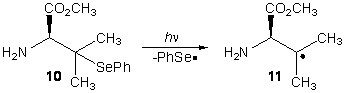Back to Table of Contents
43973-GB4
Using a Photolabile Precursor to Study Radical-Mediated Protein Damage
K. Nolan Carter, University of Central Arkansas
Oxidative
damage of proteins is believed to involve free radical intermediates. This damage process can be initiated by
reactive oxygen species such as hydroxyl radical. Protein radicals thus produced can undergo
subsequent reactions which are often complex and may result in transfer of
damage to other biological molecules such as nucleic acids. A problem associated with the direct study of
this damage process is that hydroxyl radical is highly reactive and can produce
multiple radicals from a given amino acid within a polypeptide chain. Since this is possible at every amino acid
within a polypeptide, many protein radicals can result from attack of hydroxyl
radical. This hinders direct study of specific
protein radical intermediates involved in this process. The objective of this research was to develop
photochemically active amino acids which generate the same radicals produced by
hydroxyl radical, but in a selective fashion.
It was envisioned that these photochemically active amino acids would
contain a photolabile group which would be cleaved upon photolysis to generate
amino acid radicals. Radicals such as 1, derived from the reaction of
hydroxyl radical with valine can be selectively generated under mild conditions
via this approach. We have successfully
produced a photolabile precursor for a primary valine radical, the first such
example of a compound of this type.

Since
alkyl aryl selenides are known to undergo facile C-Se bond homolysis upon UV
irradiation, the phenylselenyl group was chosen as a photolabile group. Phthaloyl protected diastereomeric valine radical
precursors 4 and 5 were prepared from the corresponding diastereomeric bromides (2,3) by treatment with benzeneselenol in the presence of cesium carbonate. This method is superior to more conventional
methods for introduction of the phenylselenyl group such as diphenyl
diselenide/sodium borohydride. Removal
of the phthaloyl protecting group with methanolic hydrazine afforded radical
precursors 6 and 7 as a 2:1 mixture of diastereomers. This compound was intended to generate a
monomeric valine radical that would serve as a model for the analogous valine
radical produced within proteins by hydroxyl radical.

Upon
photolysis at 350 nm, this compound is converted to products consistent with
the intermediacy of radicals 8 and 9.
Detailed characterization of photolysates by BSTFA derivatization
followed by GC/MS analysis is in progress.

Studies toward the synthesis of
tertiary radical precursor 10 have
also been conducted. This compound is
intended to produce tertiary valine radical 11.
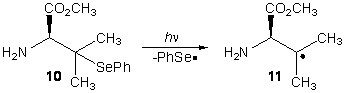
Due to the scarcity of available
methods for the synthesis of tertiary alkyl aryl selenides, synthesis of
tertiary radical precursor 10 has yet
to be completed. Having attempted
unsuccessfully to prepare this compound from substitution of the corresponding
alkyl halide, we are currently attempting to complete the synthesis via 1,4-addition of benzeneselenolate to alkene 12 to produce protected radical
precursor 13.
Back to top