

46724-G5
Ab Initio Study of the Potential Dependence of the Surface Structure and Reactivity of Doped Ceria Anodes for Use in Direct Hydrocarbon Solid Oxide Fuel Cells
This project utilizes quantum chemical methods to probe the activity and stability of mixed oxides for use as anode electrocatalysts for direct hydrocarbon oxidation in a solid oxide fuel cell (SOFC). Over the first year of the grant period, our efforts have focused on determining how the composition and structure of Pd and Zr doped Ceria surfaces alters the thermodynamics and kinetics of the initial C-H activation reaction step, specifically using methane as a probe hydrocarbon molecule. The DFT+U method has been used to model the electronic structure of the doped ceria surface, allowing for proper representation of the reduced states. We have demonstrated that both the reaction energy and activation barrier for the key C-H activation step correlate with the reducibility of the surface. Furthermore, we have shown that methane activation over the Pd-doped ceria surface occurs through C-H dissociation over surface oxygen atoms, and that the role of Pd in accelerating this process is to provide a more reducible metal center in the surface. Calibration of the choice of U parameter in the electronic structure method has enabled us to quantitatively evaluate the impact of dopant metal on surface reducibility. We have used electronic structure modeling to elucidate the trade-offs in design between being reducible enough to activate methane over a low reaction barrier while not requiring an extensive input in energy to recreate the oxidized surface. Our results, therefore, have provided insight into the competing design factors dictating the choices for optimizing the electrocatalytic activity of direct hydrocarbon SOFC anodes. This initial work has results in a single published paper. Future directions include further investigation into the methane activation mechanism and evaluation of the energetics of a complete catalytic cycle to produce carbon dioxide and water.
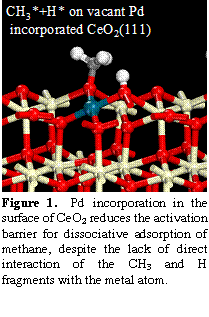
Figure SEQ Figure \* ARABIC 1. Pd incorporation in the surface of CeO2 reduces the activation barrier for dissociative adsorption of methane, despite the lack of direct interaction of the CH3 and H fragments with the metal atom.
 In addition to our focus on
catalytic activity, we have developed an approach to using quantum chemical
methods to determine the stability of the mixed oxide surface under SOFC
operating conditions. Initial results
indicate that the Pd-incorporated ceria surface is stable only under extreme
SOFC operating conditions, and therefore that the search for an active
formulation that is stable against particle growth and segregation remains
incomplete. We are currently preparing a
second manuscript on the stability of ceria based mixed oxides as a function of
reaction conditions. Future work will
consider other noble metal dopants in a search for a formulation
that may be both active and stable under SOFC operating conditions.
In addition to our focus on
catalytic activity, we have developed an approach to using quantum chemical
methods to determine the stability of the mixed oxide surface under SOFC
operating conditions. Initial results
indicate that the Pd-incorporated ceria surface is stable only under extreme
SOFC operating conditions, and therefore that the search for an active
formulation that is stable against particle growth and segregation remains
incomplete. We are currently preparing a
second manuscript on the stability of ceria based mixed oxides as a function of
reaction conditions. Future work will
consider other noble metal dopants in a search for a formulation
that may be both active and stable under SOFC operating conditions.
The funding of this ACS-PRF G proposal has enabled my group to initiate this research into the SOFC technology. This project has also enabled us to develop an additional capability in the use of the DFT+U methodology. This capability and my developing expertise in the catalytic chemistry of ceria and other rare earth oxides has lead to my inclusion in two current Energy Frontier Research Center proposals to be submitted in October 2008 to the Department of Energy. I also gave an invited talk at the Gordon Research Conference on Fuel Cells in July 2008 that included the results of this project.
Adam D. Mayernick is completing his
Ph.D. thesis research on this project under my advisement. The grant funds are currently allocated to
support his tuition and stipend during the 2008-2009 school year. Adam's initial work on this project was
supported by departmental start-up funds.
Adam has developed the ability to perform electronic structure calculations
and to apply them to this challenging catalyst problem. Additionally, he has completed coursework in
Electrochemical Engineering and is developing an expertise in alternative
energy technologies. Adam is first
author on the first publication coming from this project. He has presented the results of this research
with a poster at the national meeting of the Electrochemical Society in