46743-B3
Homoleptic and Heteroleptic Copper(I) Phenanthrolines: Sterically Enhanced Excited States
SEQ CHAPTER \h \r 1Background:
This project focuses on nine unique phenanthroline derivatives designed to create sterically congested and electronically activated heteroleptic and homoleptic copper(I) complexes. This modification should create longer-lived charge separation in the excited state as a model for creating copper(I) based solar energy conversion devices superior to ruthenium based devices.
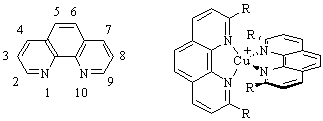
Figure 1: (Left) The
numbering sequence for the 1,10-phenanthroline ligand.
(Right) A typical structure for
a bis-phenanthroline copper(I) complex with R groups
in the
important 2 and 9 positions.
Previous data from the grant
proposal demonstrated that derivatives of the 3,4,7,8-tetramethyl-1,10-phenanthroline
(tmp) enhance the luminescent
intensity of the subsequent copper complex compared to the unmethylated
derivatives. Therefore, we dedicated the
majority of our efforts this year towards the synthesis of alkyl derivatives of
the tmp ligand using the procedure for
the unsubstituted phenanthroline in Figure 2 below:
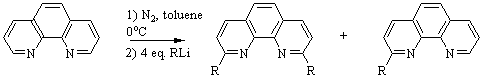
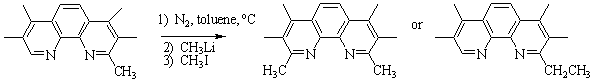
Figure 2: Reaction scheme
for the nucleophilic aromatic substitution on 1,10-phenanthroline
with excess lithium reagent.1
Products recovered are both the mono and di-substituted compounds which
are separated via column chromatography.
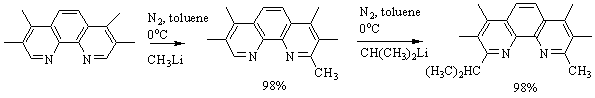
Results: The reactivity of the alkyl lithium
reagents was significantly different using 3,4,7,8-tetramethyl-1,10-phenanthroline. Following the reaction above, tmp underwent a unique, single
substitution with CH3Li creating 2,3,4,7,8-pentamethyl-1,10-phenanthroline
(pmp) in a quantitative yield. Using this anomaly, we efficiently
synthesized the asymmetric 9-isopropyl-2,3,4,7,8-pentamethyl-1,10-phenanthroline
(Figure 3) from pmp starting
material.
Figure 3: Reaction scheme
for the creation of asymmetric 9-isopropyl-2,3,4,7,8-pentamethyl-1,10-phenanthroline
(ippmp)
Surprisingly, neither CH3Li
nor C(CH3)3Li were found to
react with the pmp starting material
in the manner in which the CH(CH3)2Li reagent was found
to do. We hypothesized that the t-butyllithium was experiencing a steric
hindrance towards the reaction site that was not encountered by the smaller
isopropyl reagent. Since same conclusion
would not be feasible for the methyllithium; we initially believed the five
methyl groups were deactivating the ring towards a nucleophilic attach. This would account for the lack of reactivity
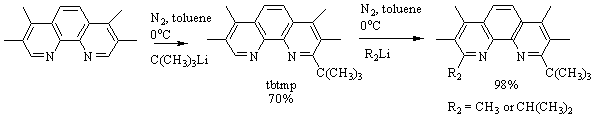
of the “weakest” nucleophile of the series CH3Li.2 Investigating the t-butyllithium reactivity identified
that only the mono-t-butyl derivative
(70% yield) was collected with no evidence of a
di-substituted product being created.
This supported our conclusion that the t-butyl group was too large to
occupy both the 2 and 9 positions with methyl groups at the 3 and 8. Once again, we used the high yield of the
monosubstituted derivative to create asymmetric compounds as seen in Figure 4.
Figure 4: Reaction scheme
used to create the 2-t-butyl-3,4,7,8-tetramethyl-1,10-phenanthroline (tbtmp). This starting
material was used to make both the 2-t-butyl-9-isopropyl-3,4,7,8-tetramethyl-1,10-phenanthroline
(tbiptmp) and the 9-t-butyl-2,3,4,7,8-pentamethyl-1,10-phenanthroline
(tbpmp).
These two new monosubstituted and
three new asymmetric ligands have been characterized by 1H and 13C-NMR,
GC-MS and melting point. Pending data
will be from submission for CHN analysis using a contract lab. Future work will be to increase production of
the asymmetric ligands for creating the homoleptic and heteroleptic copper(I) complexes.
Additional future work will be the
unanswered question of how to make the 2,3,4,7,8,9-hexamethyl-1,10-phenanthroline
(hmp). It is clear the neither tmp nor pmp will react
with methyllithium alone to create the hmp. If the methyllithium will not act as a
nucleophile, perhaps it will act as a base (Figure 5). Alternatively, another direction will be the
use of N,N,N',N'-tetramethylethylenediamine (TMEDA) to
increase the reactivity of the methyllithium to increase the likelihood of a
nucleophilic attack at the remaining alpha-carbon.3
Figure 5: Reaction scheme
as an attempt to create the
2,3,4,7,8,9-hexamethyl-1,10-phenanthroline.
This project also involves the
supervision of undergraduates4 as a part of the project as per the
PRF mission for students
“...to become involved in advanced investigative research
activities, in preparation for continued study in graduate school or
employment.”5
This ongoing project will be the topic of both oral presentations
and poster presentations from the current students during this academic
year.
References: 1) a)
Dietrich-Buchecker, C.O.; Marnot, P.A.; Sauvage, J.P. Direct Synthesis of
Disubstituted Aromatic Polyimine Chelates. Tetrahedron
Lett., 1982, 23, 5291
2) March, J., Advanced Organic Chemistry: Reactions, Mechanisms,
and Structure, 4th ed., 1992, Wiley, 3) The Chemistry of Organolithium Reagents, Zvi Rappoport, Wiley, 4) Project Undergraduates from
grant proposal to current year: Previous:
Tim Berto (‘07), Ph.D. program at University of Michigan; Laura Kubista (‘08),
Ph.D. program at SUNY at Stony Brook; Steve Kraft (‘08) Ph.D. program at Purdue
University. Current: James Lindlof (‘09) and Ian Klein (‘10).
5)http://portal.acs.org/portal/acs/corg/content?_nfpb=true&_pageLabel=PP_TRANSITIONMAIN&node_id=1263&use_sec=false&sec_url_var=region1
(PRF website accessed 09/03/2008
)