45822-B7
Ordered Arrays of Polyphenols by Tandem Reactions
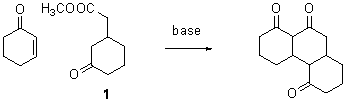
During the summer of 2007 Amin Khoshnevisan and Zak Page worked on the Kekulene project. Amin tried many different approaches to the condensation reaction shown, but was unable to improve on the previously best yield of 10%. However, he did succeed in doing the reaction many times and thus increased our stock of this compound.
Zak concentrated on
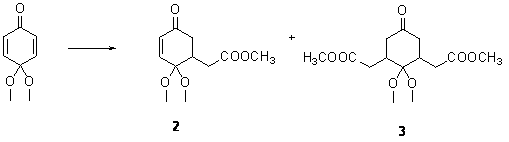
the synthesis of the two ketoesters necessary for
further elaboration of this condensation into extended systems. Compounds 2 and 3 are previously
unknown. Zak was able to make both in
moderate yields. We are not happy with
the yields yet so have not reported this chemistry other than in posters at
meetings, but both compounds are well characterized and have nearly first order
NMR spectra.
During the summer of 2008 Nate Eldredge continued Amin's work, with similar results. Our best conditions are sodium hydride in
THF. In this reaction we are always
plagued by Baylis-Hillman reaction of cyclohexenone with itself, catalyzed, we presume, by methoxide given off by the condensation reaction. We have concluded that the way to improve
the condensation is to make sure that the leaving group cannot catalyze the Baylis-Hillman reaction.
Accordingly, Mark Weir set out to make the tert-butyl ester corresponding to
1.
There is only one report of this compound in the literature, by a
somewhat complex route. Although only a
freshman, Mark developed three new routes to this compound. So far the yields are low, but we are working
on improving all three routes and will focus in one whichever becomes most
promising. After we have conditions
worked out, we will apply them to the synthesis of the tert-butyl analogs of 2 and 3, and also attempt the first condensation
reaction using the new tert-butyl
ester to see if the yield improves.
This work has been reported at the National Organic
Symposium in June of 2007, the ACS meeting in March of 2008, and the Reaction
Mechanisms Conference in June of 2008, as well as several seminars given by the
PI at other institutions.