

46943-G3
Investigation of O2 Activation with Metallodendrimer Models of Copper Monooxygenases
Aliphatic hydrocarbons are underutilized as chemical feedstocks because making useful derivatives requires catalysts that can oxidize C-H and C=C bonds efficiently and selectively with minimal energy requirements. Few of the numerous approaches chemists have investigated to date have produced an economically viable solution for derivation of these abundant components of crude oil. Nature has constructed a number of metalloenzymes that can oxidize unreactive bonds under mild conditions with unprecedented selectivity and high turnover numbers at rates near the diffusion limit. Researchers have constructed biomimetic coordination complexes to reproduce biological oxidation reactions with synthetic systems; however, none of these active site models exhibit catalytic chemistry reminiscent of the enzymes. We are interested in improving models for oxidation chemistry by optimizing metal-oxygen reactivity with macromolecular effects.
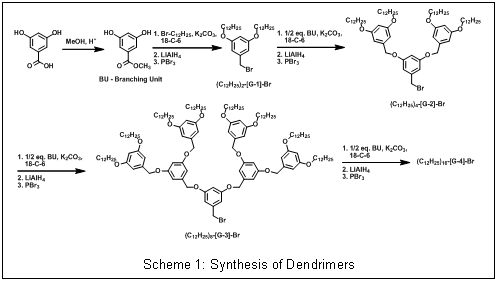
Scheme 1: Synthesis of Dendrimers |
 Our research involves
the design and synthesis of dendritic copper
complexes inspired by monooxygenases, and their
reactions with dioxygen. Dendrimers have unique
properties capable of producing nanoenvironments
conducive to forming dioxygen adducts that are inaccessible using traditional
small molecule model complexes. Our initial work focused on constructing dendronized b-diiminate (BDI) ligands that closely replicate existing
small molecule systems capable of supporting 1:1 Cu:O2
adducts; however, we have recently expanded the scope of ligands that we are
investigating based on recently reported small molecule model complexes that
exhibit promising O2 activation chemistry.
Our research involves
the design and synthesis of dendritic copper
complexes inspired by monooxygenases, and their
reactions with dioxygen. Dendrimers have unique
properties capable of producing nanoenvironments
conducive to forming dioxygen adducts that are inaccessible using traditional
small molecule model complexes. Our initial work focused on constructing dendronized b-diiminate (BDI) ligands that closely replicate existing
small molecule systems capable of supporting 1:1 Cu:O2
adducts; however, we have recently expanded the scope of ligands that we are
investigating based on recently reported small molecule model complexes that
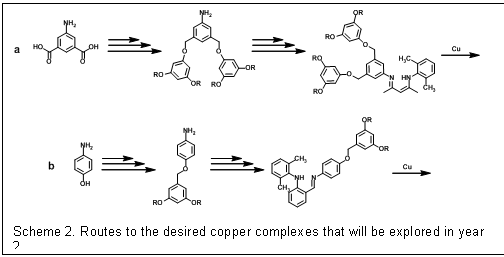
exhibit promising O2 activation chemistry.The efforts during the first year on this project have focused on building the necessary dendrimers to prepare the desired copper complexes. The researchers on this project have developed and refine synthetic methods for making poly(benzyl ether) dendrimers (Scheme 1). Poly(benzyl ether) dendrimers are frequently employed for catalysis because 3rd generation derivatives provide maximum dendritic encapsulation with the most facile synthesis. These aromatic dendrimers are attractive for modeling enzymatic active sites because the repeat units resemble amino acids like phenyl alanine that often form hydrophobic pockets around enzyme active sites. Despite these favorable properties, benzylic hydrogens present in these dendrimers are susceptible to abstraction by metal-bound oxygen adducts and radical species often observed in biominetic oxidations, so in our initial model complexes we are using a ligand construct that will direct the dendrimer away from the copper active site. While building ligands with to multiple dendrons branching off the ligand in close proximity to the active site may be more likely to afford models with the desired properties (Scheme 2a), we are completing the construction a proof-of-concept system based on commercially available 4-aminophenol (Scheme 2b). While dendrimer and ligand synthesis has dominated the first year of the project, we anticipate making copper complexes and testing their O2 reactivity during year 2.
Scheme 2. Routes to the desired copper complexes that will be explored in year 2.
|
 Nature uses monomeric copper enzymes to hydroxylate
aliphatic hydrocarbons; however, none of the synthetic models reported to date
can effectively exploit this chemistry to provide efficient oxidation
reactions. Dendritic ligands represent a promising
strategy to provide synthetic systems the properties necessary to access copper
superoxide species. The initial phase of research has been successful in making
dendrimers needed to prepare ligands and the
corresponding copper complexes necessary to study oxygen activation chemistry. When
the synthesis of the desired copper complexes is complete, we will begin to
explore their reactivity with oxygen and any potential biomimetic
C-H oxidation chemistry.
Nature uses monomeric copper enzymes to hydroxylate
aliphatic hydrocarbons; however, none of the synthetic models reported to date
can effectively exploit this chemistry to provide efficient oxidation
reactions. Dendritic ligands represent a promising
strategy to provide synthetic systems the properties necessary to access copper
superoxide species. The initial phase of research has been successful in making
dendrimers needed to prepare ligands and the
corresponding copper complexes necessary to study oxygen activation chemistry. When
the synthesis of the desired copper complexes is complete, we will begin to
explore their reactivity with oxygen and any potential biomimetic
C-H oxidation chemistry.