47135-AC1
Hydrolase Engineering by Circular Permutation
Our studies over the last 12 months have focused on evaluating the impact of circular permutation on the structure and function of lipase B from Candida antarctica.
Evaluating the structural dynamics of the termini regions in cp283.
We successfully overexpressed and purified a series of lipase variants with cysteine mutations near the N and C termini of cp283 (N: V286C, G288C; C: V272C, A275C, A279C). For comparison, the same substitutions were introduced in wild type CALB. After demonstrating that the individual mutations do not interfere with the enzymes' catalytic function, we labeled the thiol side-chains of individual mutants with BODIPY 507/545 (Invitrogen). BODIPY's short C2-linker provides a tight connection between protein and fluorophor for the proposed fluorescence anisotropy (FA) experiments. Steady-state FA experiments and time-resolved anisotropy decay experiments are currently ongoing in the lab.
Exploring the functional significance of the new termini regions by truncation.
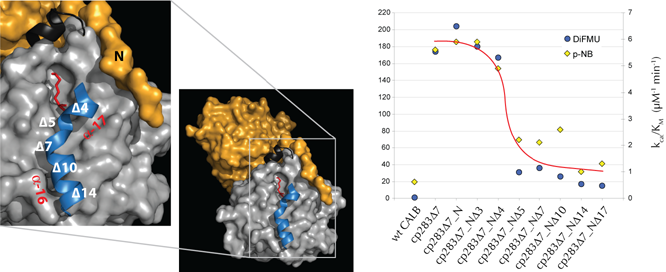
Separately, we have completed a series of experiments, studying the effects of N and C-terminally truncated cp283Æ7 on hydrolase function. The elimination of up to 5 N-terminal residues in cp283Æ7 resulted in no significant activity change for two test substrates (Fig. 1) as predicted based on the terminus' orientation away from the active site as seen in the phosphonate inhibitor-bound crystal structure of cp283Æ7. In contrast, the stepwise truncation of the 17 invisible residues of the C-terminus indicates that while the elimination of amino acids in a17 are well tolerated, the removal of additional residues leads to a rapid decline in activity (Fig. 1). We attribute the activity change, going from cp283Æ7-NÆ4 to Æ5 (elimination of Leu278), to the residue's role in capping a16. The data suggest that a16, despite of showing no electron density in the x-ray structure, assumes an ordered secondary structure and contributes towards catalysis.
Functional re-design of cp283Æ7.
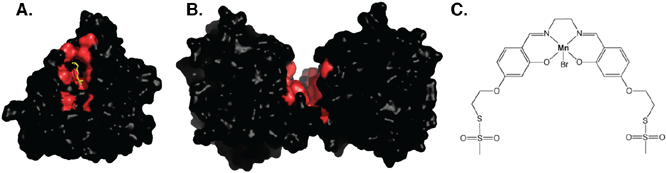
A very exciting aspect of our CALB engineering has been the insight into the structural consequences of circular permutation. The crystal structure of cp283Æ7 revealed not only major changes in the active site topology but also a dramatic reorganization of the protein's quaternary structure, leading to novel surface features. Circular permutation has widened the active site access tunnel into a cleft and has created an entirely new channel at the protein dimer interface (Fig. 2A/B). Besides raising interesting new questions (and possibilities) regarding the evolutionary relevance of circular permutation, this system offers a unique opportunity to explore the creation of biocatalysts with new and multiple functionality.
In an attempt to redesign the active site cleft of cp283Æ7, we are in the process of introducing a manganese center for enantioselective sulfoxidation and epoxidation. Metallosalen complexes are known tools in asymmetric catalysis. A prominent example is Jacobsen's catalyst for the efficient enantioselective epoxidation of olefins (ee >99%) in the presence of sodium hypochlorite. Despite its high effectiveness, intrinsic limitations of homogeneous catalysis and the undesirable use of hypochlorite as oxidizing agent have encouraged the development of new catalysts featuring easier recovery and milder oxidizing conditions. Lu and coworkers (JACS, 2004, 126 p.10812) recently reported the synthesis and characterization of an artificial Mn-protein prepared by anchoring a non-chiral Jacobsen-like Mn(III) complex (Fig. 2C) via two cysteine residues engineered in the Heme site of apo sperm whale myoglobine. Compared to the poor activity and negligible enantioselectivity exhibited by the Mn complex itself, the artificial Mn-myoglobine showed increased turnover number and enantioselectivity for the sulfoxidation of thioanisole in the presence of H2O2 as oxidizing agent.
Docking studies to the cp283Æ7 crystal structure indicate a good fit of the Mn complex in the newly created active site cleft, allowing coordination of Mn to active site His224. Mutagenesis of Trp104Cys and Gln157Cys would create two properly positioned anchor points for the metal ligand via disulfide bonds. At this point, we have successfully synthesized the metal ligand and introduced the necessary site-directed mutations in cp283Æ7 (Trp104Cys, Gln157Cys). Upon completion of the protein mutant overexpression in Pichia pastoris, we will couple the metal-ligand complex to the reduced enzyme and investigate the system's activity towards enantioselective sulfoxidation of thioanisole.
Figures
Figure 1: Crystal
structure of cp283Æ7 dimer (orange & grey subunits). The insert shows a
close-up view of one of the two active sites (bound phosphonate inhibitor shown
in red stick) with a model of the invisible C-terminus in blue (the model is
based on the wild type CALB structure). The effects of incremental truncation
at the C-terminus (a16 & a17) on activity were measured with p-nitrophenol
butyrate (p-NB) and 6,8-difluoro-4-methylumbelliferyl octanoate (DiFMU).
Figure 2: Functional redesign of cp283Æ7. A.) Enzyme
engineering has converted the active site access tunnel of CALB into a cleft
(highlighted in red with phosphonate suicide inhibitor shown in yellow stick).
B.) cp283Æ7 dimer, revealing the channel at the dimer interface (highlighted in
red). C.) Structure of the Mn-ligand complex (N,N'-bis(4-(2-methanesulfonylthioethoxy)salicylidene)-1,2-ethanediamino-manganese(III)
bromide).