45891-AC7
Controlled Polymerization of Renewable Cyclic Esters: Catalyst Design and Polymerization Mechanisms
Decreased reliance on petroleum feedstocks for plastics production will be an important contributor to sound long-term national and international energy policy. As such, the petroleum research field will ultimately need to adapt to this inevitable shift from current practices. An attractive strategy is to develop new methods for the synthesis of polymers with useful properties from renewable starting materials. Towards this end, new and innovative methods are sought for the conversion of molecules provided by plants into compounds that can be catalytically transformed into sustainable plastics. We have taken an interdisciplinary approach that integrates chemical synthesis and structural definition of new catalysts, monomers, and polymers, with particular emphasis on mechanistic studies of polymerization catalysis via synergistic use of experimental and theoretical methods. Specific aims are to (i) develop a new class of polymerization catalysts based on a recently proposed activated monomer mechanism, (ii) uncover important and fundamental mechanistic information concerning these catalysts, and (iii) develop syntheses of new polymeric structures with useful properties from new monomers derived from agricultural products.
In work supported by the ACS-PRF, we have synthesized and characterized two zinc(II) bis(phenolato)amine complexes L2Zn2 (L = methylamino-N,N-bis(2-methylene-4,6-di-tert-butylphenolato), L1, or methylamino-N,N-bis(2-methylene-4-adamantyl-6-tert-butylphenolato), L2) and studied their e-caprolactone (CL) polymerization activity and kinetics. X-ray crystallographic and 1H NMR studies, including NOESY and PGSE experiments, provided insight into the solid and solution state structures, respectively, as well as evidence for the catalytically active species responsible for the ring-opening polymerization of CL. Additionally, solution polymerizations and kinetics experiments involving (L1)2Zn2 in the presence of benzyl alcohol (BnOH) were performed to elucidate the influence of catalyst structure, solvent, and the concentration dependence of the catalytically active species, CL, and BnOH on the rate and control of poly-e-caprolactone (PCL) formation. The structural, polymerization, and kinetics data support equilibria involving both mononuclear and dinuclear forms of (L1)2Zn2 as well as a monomer-activated route to PCL.
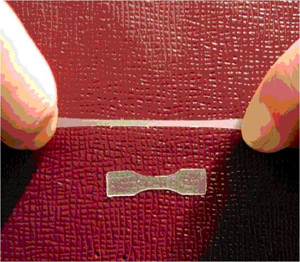
In separate work, an alpha-omega-functionalized polymenthide was synthesized by the ring-opening polymerization of menthide in the presence of diethylene glycol with diethyl zinc as the catalyst. Termination with water afforded the dihydroxy polymenthide. Reaction of this telechelic polymer with triethylaluminum formed the corresponding aluminum alkoxide macroinitiator that was used for the controlled polymerization of lactide to yield biorenewable polylactide-b-polymenthide-b-polylactide triblock copolymers. The molecular weight and chemical composition were easily adjusted by the monomer-to-initiator ratios. Microphase separation in these triblock copolymers was confirmed by small angle x-ray scattering and differential scanning calorimetry. A representative triblock was prepared with a hexagonally packed cylindrical morphology as determined by small angle x-ray scattering, and tensile testing was employed to assess the mechanical behavior. Based on the ultimate elongations and elastic recovery these triblock copolymers behaved as a thermoplastic elastomers, as illustrated in the Figure.
Figure. Illustration of the
thermoplastic elastomeric behavior of a sample of the biorenewable polylactide-b-polymenthide-b-polylactide triblock copolymer.