46671-AC6
Interactions Between Small Hydrocarbons and Water: Clathrate Formation and Inhibition
There is many times more carbon in clathrate
hydrates on earth than all other known hydrocarbon sources. Understanding how
these materials form can make the difference between clathrates being an energy
savior or a potential environmental disaster. The primary tools used to date to
determine the events leading to clathrate nucleation and growth are x-ray,
neutron scattering and NMR spectroscopies. X-ray and neutron scattering are
limited because they detect seed crystals that have already grown to at least a
few nm. On a molecular scale, a few nm is very large. NMR spectroscopy is
capable of detection on the molecular level, but requires a relatively high
concentration, so limited for detection of the molecular-level nucleating
interactions. Up to this point, vibrational spectroscopy has not been used in
aqueous systems due to the opacity of aqueous samples in the hydrogen bonding
region, yet vibrational spectroscopy is an exquisitely sensitive probe of
interactions between water and other molecules including the guest in a
clathrate structure. This project surmounts the opacity problem with two strategies.
(1) Water and the interactant are dispersed in an infrared transparent medium.
This isolates the clusters avoiding the opacity issue. Previous work using this
strategy dispersed water in CCl4. Clathrates form at high pressure
(10-70 bar), ideal for using supercritical solvents – Ar, Xe, or Kr – as the
isolating medium. (2) The second strategy is to use a surface sensitive
technique and examine the interaction between the guest and solid water. In
this first year, progress has been made on both fronts, each of which is
outlined below.
  Figure 1: Burning clathrate on the laboratory bench. |
Figure 2: Propane in supercritical At at 0 °C in the CH asymmetric stretch region. |
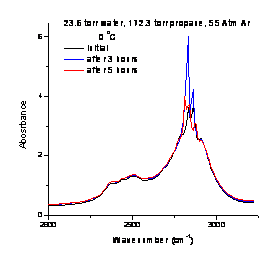
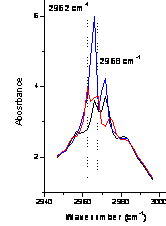
The first task was to design an optical cell capable of withstanding an internal pressure of at least 100 atm. Since such cells are not commercially available a cell was designed, built and tested. Figure 1 contains a photograph of a clathrate formed in the cell and ignited. Interactions between water and the guest (propane) were probed using infrared absorption. Figure 2 shows the CH vibrational spectrum of propane in supercritical Ar at 0 °C. Note the broad featureless envelope of the rotational bands on the asymmetric stretch and combination bands. In contrast, (Figure 3) in the presence of water, the rotational structure is quenched resulting in distinct vibrational resonances. Further work, including reducing the propane:water ratio, is needed to definitively attribute the altered vibrational structure to clathrate formation rather than propane-water interactions limiting the rotational motion of propane. It is clear that the presence of water significantly alters the rotational structure of propane.
Figure 3: Propane and water in supercritical Ar at 0 °C. Inset at right shows a magnified view of the CH asymmetric stretch region. |
|
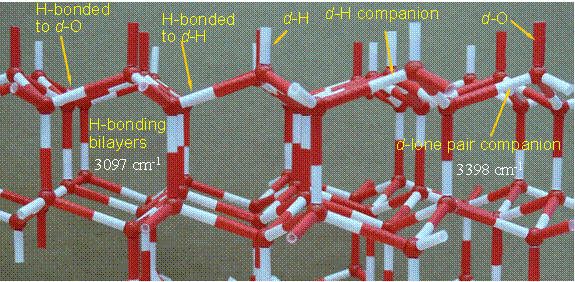
Work on the ice surface has resulted in definitive assignment of vibrational modes due to (a) bilayer connecting hydrogen bonds that reflect the ice surface morphology, (b) donor modes associate with dangling lone pairs (aka d-O), and (c) and the OH stretch associated with the dangling OH (d-OH). These are illustrated in Figure 4. Of the assigned modes, (a) and (b) are new while enhanced information has been gained for (c). Identification of these modes is important for understanding clathrate formation: interaction in a hydrogen-bond accepting configuration affects the d-OH intensity, interaction in a hydrogen-bond donating configuration affects the d-O companion intensity. It is expected that dosing the ice surface with propane will thus identify whether the fundamental water-propane interaction is via the electronegative carbon with the d-OH or via a hydrogen bond with the dangling lone pair. Further the intensity of the bilayer stitching OH bonds will diagnose disruption of the ice surface morphology that must occur if the guest molecule is to become surrounded by water in a clathrate structure.
The goals for the coming year are to use the more polarizable supercritical solvents, Kr and Xe, to increase the water concentration and to extend the reported propane work to ethane and methane. The ice surface work will be extended by dosing the surface with methane, ethane and propane.