

43905-G1
Phosphoranyl Radical-Mediated Deoxygenative Transformation of Carbonyl Groups
Progress Report for ACS PRF 43905-G1
Advance in Au catalysis
With the partial financial support of this grant, we have made significant progress in Au catalysis for the last year. A total of five papers including four JACS communications and one OL were published.
Our study has been focused on further developing Au catalysis into synthetically highly useful methods. Cycloaddition reactions are of particular importance in organic synthesis due not only to one-pot formation of multiple bonds but also to their bimolecular nature. However, Au chemistry has been of limited use in this area.
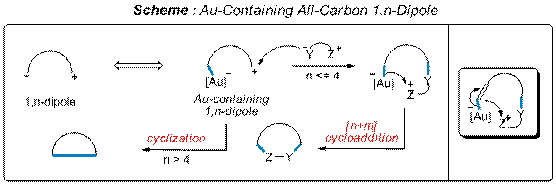
We have developed a novel concept of Au-containing all-carbon 1,n-dipoles and explored the reactivities of these species in three studies (JACS 2008, 1814; JACS 2008, ASAP; J. Organomet. Chem. 2008, accepted). The key feature in this design is that the anion end of the 1,n-dipole is capped by a Au complex, thus attenuating its reactivity and allowing the cation end to initiate the stepwise cycloaddition process (Scheme 1).
Lately, our study on ĎAu-Catalyzed Synthesis of
5,6-Dihydro-8H-indolizin-7-ones from N-(Pent-2-en-4-ynyl)-b-Lactams' was just accepted for publication at Oganic Letters.
These studies have significant impact on the field of Au
catalysis as it continues to be one of the hot topics in organic chemistry.
These studies have been reviewed favorably and published in highly reputed
journals; moreover, the novel concepts developed help to push the frontier of
Au catalysis further.
†††††††††††
Impact on PI's
career and students' education. The PI's career has substantially
benefited from this grant. With its support the PI has published many studies
in the past two years in Au catalysis. These publications have attracted
significant attention in the synthetic community. As a result, the PI was
awarded NSF CAREER (2008), Mousel-Feltner Award for
Excellence in Research and/or Creative Activity (UNR. 2008), Amgen Young
Investigator's Award (2008) and Thieme Journal Award (2007). Moreover, the PI has
been invited to lecture in various Universities (UCLA, With the help of
this grant, graduate students and postdocs have progressed
very well in their education in the lab and stayed highly productive. Meng Yu, the first graduate student, just graduated with a
M. S. degree and will join a pharmaceutical company in
 In
addition, we also developed methods generating Au carbene-containing
1,3-dipoles including all-carbon 1,3-dipoles (Org. Lett. 2007, 9, 4627) and carbonyl ylides (JACS 2008, 130, 6944). These
novel approaches not only reveal unprecedented chemistry but also offer highly
efficient access to benzyl-protected phenol derivatives via a formal [3+3]
approach and various highly functionalized bicyclo[3.2.0]heptanes.
In
addition, we also developed methods generating Au carbene-containing
1,3-dipoles including all-carbon 1,3-dipoles (Org. Lett. 2007, 9, 4627) and carbonyl ylides (JACS 2008, 130, 6944). These
novel approaches not only reveal unprecedented chemistry but also offer highly
efficient access to benzyl-protected phenol derivatives via a formal [3+3]
approach and various highly functionalized bicyclo[3.2.0]heptanes.