

46271-AC4
The Oxygen Reactivity of Flavoenzymes: A Structural Approach
Flavin-containing monooxygenases (FMOs) catalyze the oxygenation of many nitrogen- sulphur-, phosphorous-, selenium-containing nucleophilic compounds using molecular oxygen and NADPH as substratesá (Figure 1). These enzymes have become subject of intensive research because they are involved in the metabolism of a wide range of drugs, carcinogens and other xenobiotics.
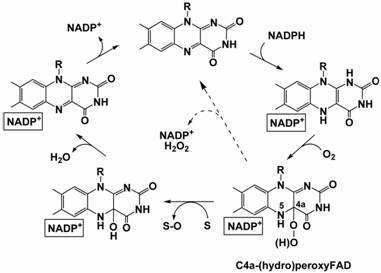
Figure 1. The catalytic cycle of FMO.
The FMO catalytic cycle is complex and involves several steps (Figure 1). The fundamental property of these enzymes is their ability to stabilise the flavin-hydroperoxide. Stabilisation of the intermediate requires the presence of NADP+, which remains tightly bound to the enzyme throughout the catalytic cycle. Given these properties, it is evident that FMOs represent an ideal system to study the structural enzymology of flavin-hydroperoxide formation and, more generally, to gain insight into the reaction of flavoproteins with oxygen.
Properties of Methylophaga FMO
A key aspect of mammalian FMOs biochemistry and physiology is their association to the microsomal membranes through a C-terminal hydrophobic segment acting as a membrane anchor. This has complicated the heterologous over-expression of these proteins and their biochemical and structural characterization. Recently, a bacterial FMO from Methylophaga sp. strain Sk1 has been identified. This protein shares 31% sequence identity and several functional properties with human FMOs. In the framework of the ACS-PRF project, we have expressed and purified the protein which has allowed us to confirm that the protein tightly binds FAD, reacts with trimethylamine and similar nucleophiles, and, most importantly, appears to be able to stabilise the flavin-hydroperoxide intermediate (Figure 2).
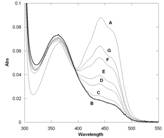
Figure 2. Absorbance spectra of bacterial FMO. Trace A represents the fully oxidized form. Trace B was obtained by the aerobic addition of an equimolar amount of NADPH and is consistent with a C4a-hydroperoxyflavin intermediate containing a small amount of oxidized enzyme (shoulder at about 450 nm) due to the intrinsic NADPH-oxidase activity. Traces C to G were sequentially recorded after 25 sec, 90 sec, 3 min, 10 min, and 18 min and show the slow conversion of the intermediate to the oxidized flavin.
Three-dimensional structure
We have solved the three-dimensional structure of bacterial FMO at 2.7 ┼ resolution using seleno-substituted protein for solution of the crystallographic phase problem. This result precisely represents the main aim for the first year of the project as indicated in the original grant application. The dimeric protein is made up of two distinct domains, a larger FAD-binding domain and a smaller NADP-binding domain. Both domains display a typical dinucleotide-binding fold and are connected through a double linker that may act as a flexible hinge.
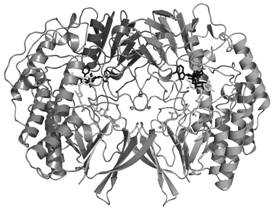
Figure 3. Overall three-dimensional structure of the Methylophaga FMO dimer.
NADP+ binds in a cleft at the interface between the two protein domains, blocking the access to the flavin and the catalytic core. The nicotinamide ring is sandwiched between the re side of the flavin and the single α-helix of the NADP-binding domain, and is roughly coplanar with isoalloxazine at a distance of 3.0-4.0 ┼. Most importantly, the nicotinamide orients its reactive C4 atom away from the flavin N5 (C4-N5 distance of 5.1 ┼) so that the NADP+ amide group is within hydrogen-bond distance from the flavin O4 and N5 (Fig. 4).
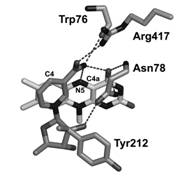
Figure 4. The active site of FMO.
Biochemical insight
Chemistry of NADPH requires that reduction of the flavin cofactor is brought about through a hydride transfer from the C4 atom of the nicotinamide ring to the flavin N5 atom. The stereochemistry observed in the FMO crystal structure does not reveal a close proximity between these two crucial atoms, and is clearly not compatible with a hydride transfer. This implies that the structure in the crystals does not correspond to the conformation in which NADPH is able to reduce the flavin. A modeling experiment in which the hypothetical coordinates of the C4a-hydroperoxyflavin are superimposed onto the flavin coordinates of the FMO structure places the two additional oxygen atoms of the C4a-adduct within hydrogen-bond distance from O2' atom of NADP+á ribose, which then would be perfectly positioned to properly stabilize this oxygenating intermediate. These considerations suggest that the conformation in FMO crystals is likely to correspond to that promoting the stabilization of C4a-hydroperoxyflavin. Our structure is a clear observation of a second fascinating functional property of NADP+ in FMO catalytic cycle, i.e. the stabilization of C4a-hydroperoxyflavin. NADP+ shields the active site and provides a proper H-bonding environment that can prolong the intermediate half-life. Therefore, NADP+ is an integral part of the catalytic machinery that promotes the monooxygenation reaction.
The scientific impact of these results is indicated by the fact that they have been published in a high-profile journal such as PNAS. This publication will also be important for the career of the two PhD students (Andrea Alfieri and Roberto Orru) who are involved in the project.