

44165-G5
Synthesis and Kinetic in situ Structural Investigations of Pt Alloy Nanoparticle Catalysts
Project objectives
Accurate measurement of size and composition of nanometer-sized particles is one of the most fundamental tasks in nanotechnology. In particular, in-situ probing of temporal changes of particle size and composition in bimetallic alloy nanoparticle catalysts is a tremendously important challenge and is essential to obtain atomic-level insight and understanding into the behavior of high surface area catalysts under operation conditions.
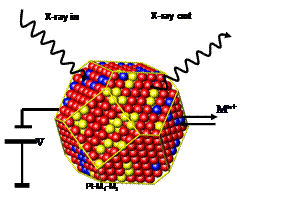
This work aims to in-situ characterize the dynamics of particle size and particle composition distributions of Pt alloy nanoparticles under electrochemical conditions. In-situ X-ray techniques (see Figure 1), such as Small-Angle X-ray Scattering (SAXS) and X-ray diffraction (XRD), are utilized to measure particle size and composition distributions as well as the nature of the alloy phases as a function of synthesis parameters and electrocatalytic reaction conditions. While in-situ SAXS focuses on the change in particle size distribution, in-situ XRD probes the cell parameters of the Pt alloys as function of time and applied potential. Both methods combined provide complementary insight in the structural and compositional stability of alloy particle electrocatalyst ensembles.
Fig 1:
Principle of in-situ real-time probing of structural transformations of
nanoparticle electrocatalysts due to metal leaching under electrified
conditions. Project
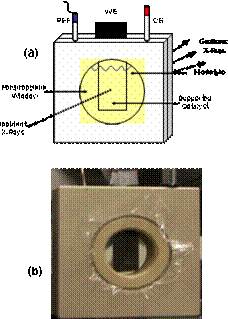
achievements to date We have designed and utilized an in-situ electrochemical XRD
and SAXS flow chamber in which Pt alloy particle electrocatalysts were
supported on a carbon paper working electrode (WE). Liquid electrolyte, counter
and reference electrode complete the electrochemical environment (Fig.2).
We have performed in situ X-ray scattering
studies (XRD and SAXS) studies of Cu rich Pt-Cu nanoparticle precursors
applying a variety of different applied potential protocols. In situ SAXS and
in situ XRD were performed at the Stanford Synchrotron Radiation Laboratory at
Beam Lines 1-4 and 11-3. We were interested in probing the structural changes that
occur if these alloy nanoparticles are subject to am applied potential in an
acidic electrolyte environment. We succeeded in probing the temporal kinetics
of the structural and compositional changes that occur during the voltammetric
preparation of the active catalyst phase. Figure 3 shows the diffraction
profiles of a Pt25Cu75 precursor catalyst as function of
time over a period of 2 hours. The catalyst is electrochemically dealloyed at a
constant potential of about 0.9V versus Ag/AgCl. The measurement indicates not
only a substantial shift of the fundamental (111) reflection; this observation
suggests a gradual expansion of the unit cell parameters due to the continuous preferential
dissolution of Cu from the Cu rich precursor. Figure 4 and Fig. 5 give the
detail information about how the integration area and peak position change over
the leaching time. Our measurements evidence that the active phase of the oxygen
reduction electrocatalyst consists of a strongly Pt enriched alloy phase.
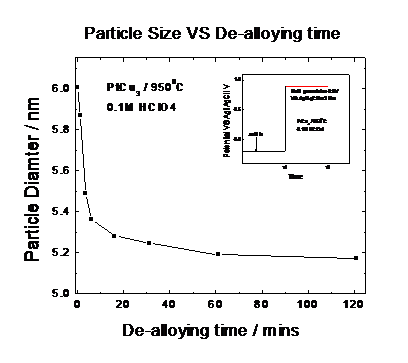
Fig 6 showed the individual time profiles of an in situ SAXS
measurement of the Pt25Cu75 nanoparticles at various time
instances. Detailed nonlinear fitting of the various Intensity-scattering
vector profiles revealed that, initially, the catalyst particles reduce their
mean particle size due to the preferential dissolution of Cu atoms. Fig 7 and
Fig.8 give the detail information how
the alloy particle size and its distrtibution changed over the experiment time
scale. Finally,
a comparative study was performed of the impact of the electrode potential on the Cu
dissolution rate from Pt25Cu75 nanoparticles and one the particle size changes. Fig. 9 and Fig. 10
report the particle size and the (111) peak integration area over time under
the different electrochemical potentials. Our results show that the more
positive the applied electrode potential is at which the nanoparticles are
being held at, the smaller the resulting particle size is and the smaller the
scattering intensity becomes indicating a loss of scattering atoms (Cu).
Fig. 2: Electrochemical-SAXS(XRD) cell Student
Training and Professional Preparation This
ACS PRF scholarship enabled the partial funding of one graduate student and one
postdoctoral student. Both student received training and professional
prepration in state-of-art materials characterizations. In Summer of 2007, a
SUMR scholarship enabled the participation of one undergraduate student in the
project.
 .
.
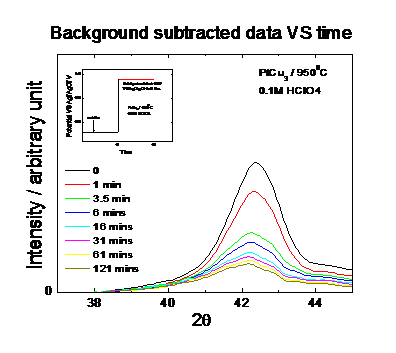
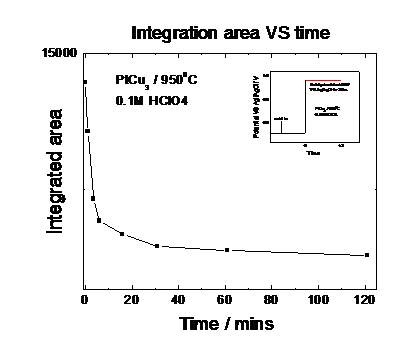
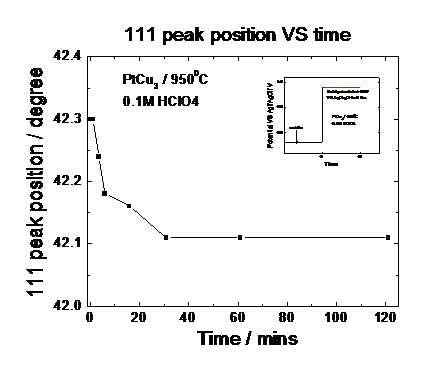
Fig. 5: Peak position over
time under electrochemical potential control obtained from Fig. 3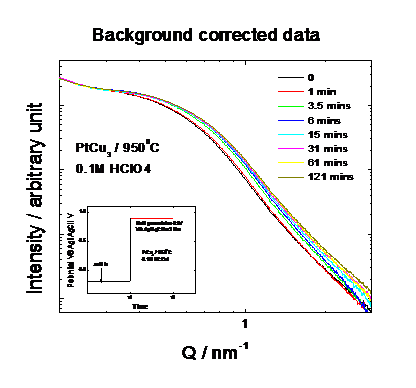
 |
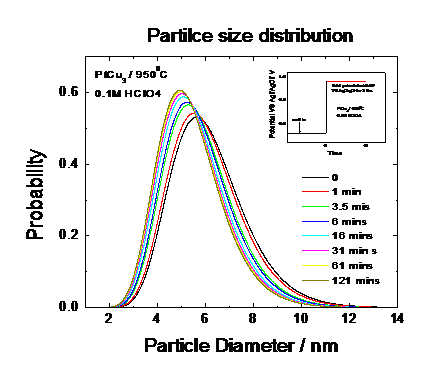 |
Fig. 8: Mean Particle Size of distributions of time under electrochemical potential control obtained from Fig. 7
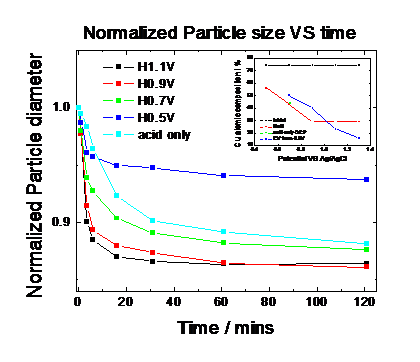 |
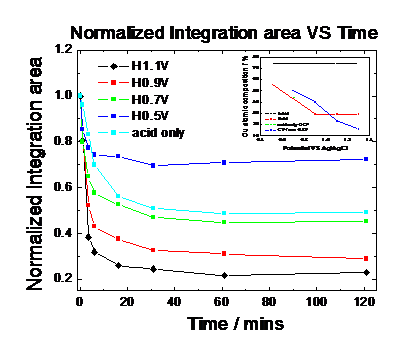 |
Fig. 10: Peak integration area at different potentials