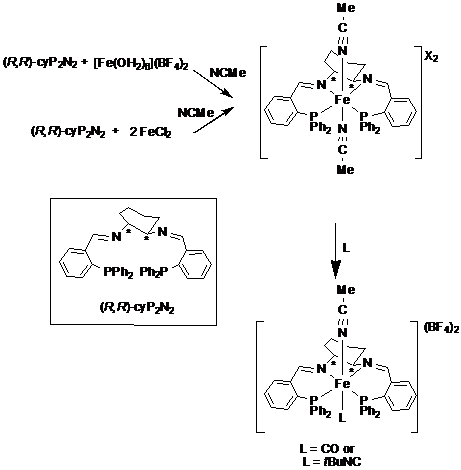46845-AC3
Polar Bond Hydrogenation Catalyzed by Iron Complexes
Tetradentate P-N-N-P ligand complexes are important in catalysis. Those based on platinum metals are catalysts for asymmetric transfer hydrogenation, direct hydrogenation, kinetic resolution of racemic alcohols, Michael addition, epoxidation and oxidation, cyclopropanation, and fluorination, both nucleophilic and electrophilic. We show in this work that those based on iron(II) are catalysts for the asymmetric hydrogenation and transfer hydrogenation of aromatic ketones. This is significant because iron-based catalysts are potentially of lower cost, toxicity and environmental impact than those of platinum metals.
Given the high activity and enantioselectivity for acetophenone transfer hydrogenation and H2-hydrogenation displayed by the ruthenium complexes RuCl2{(S,S)-cyP2(NH)2} where (S,S)-cyP2(NH)2 is the tetradentate P-NH-NH-P ligand (S,S)-{PPh2(o-C6H4)CH2NHC6H10NHCH2(o-C6H4)PPh2}, we wondered whether similar iron catalysts could be developed. The ruthenium catalyst is thought to hydrogenate ketones via a transfer of hydride from ruthenium and proton from nitrogen to the C=O bond in an outer sphere hydrogenation. Therefore the NH group is thought to be essential. Gao and coworkers reported the synthesis of the dicationic complexes [Fe(NCMe)2{ethP2N2}](ClO4)2, ethP2N2 = {PPh2(o-C6H4)CH=NCH2-}2 and [Fe(NCMe)2{ethP2(NH)2}](ClO4)2, ethP2(NH)2= {PPh2(o-C6H4)CH2NHCH2}2, but did not report X-ray structures and catalytic activity of these complexes.
Our starting point was the synthesis of well-defined complexes containing the enantiopure tetradentate ligand (R,R)-cyP2N2. We prepared a trans bis(acetonitrile) complex [Fe(NCMe)2{(R,R)-cyP2N2}](BF4)2, in excellent yield (70-90%) by two different routes (Scheme 1).
Scheme 1.
The carbonyl complex L=CO and the isonitrile complex L = tBuNC were prepared in quantitative yield by reaction with carbon monoxide (1 atm) and 2 equiv of tBuNC respectively, in acetone at 22 ºC or in refluxing chloroform (Scheme 1).
The bis(acetonitrile) complex was tested as a catalyst precursor for the H2 hydrogenation of acetophenone to 1-phenylethanol. Under 25 atm of H2 at 50 °C and in the presence of KOtBu, it showed 40% conversion with an e.e. of 27 % . This is the first report of a well-defined iron precatalyst for the asymmetric hydrogenation of ketones. The current system has a turnover frequency (TOF) of about 5 h–1 at 50 °C, somewhat less active than Casey's iron catalyst (TOF 2 h–1 at 25 °C).2 No activity was found for transfer hydrogenation catalysis.
The carbonyl complex was found to be inactive for H2 hydrogenation but it is a surprisingly efficient catalyst for the solvent transfer hydrogenation of ketones, aldehydes and imines. When the reduction of acetophenone was carried out in the presence of the isontrile complex, the e.e. reached 76% with 34% maximal conversion after 2.6 hours. Further work is required to determine the cause of this deactivation of catalysis.
In summary, the complex [Fe(NCMe)2{(R,R)-cyP2N2}](BF4)2, constitutes the first well-defined iron catalyst for the asymmetric H2-hydrogenation of acetophenone. Its modification by reaction with CO or CNtBu gives the precatalysts [Fe(CO)(NCMe){(R,R)-cyP2N2}](BF4)2, and Fe(CNtBu)(NCMe){(R,R)-cyP2N2}](BF4)2, which promote the first asymmetric transfer hydrogenation of polar bonds at room temperature, such as ketones, aldehydes and imines, with an excellent TOF (907 h–1). This catalyst system is almost as active as the most active ruthenium system. Mechanistic studies and catalyst optimization are currently underway.
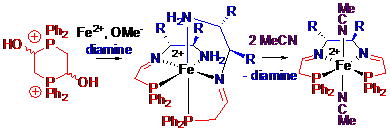
. A multicomponent template reaction utilizing an air-stable phosphonium precursor leads initially to the first enantiopure bis-tridentate iron complexes mer-[Fe(P-N-N)2]2+ in high yield (R = H, Ph; RCHCHR = cyclo-C6H10) and then to new tetradentate iron complexes trans-[Fe(MeCN)2(P-N-N-P)]2+ (R = H, RCHCHR = cyclo-C6H10; RCHCHR = C6H4) (Scheme 2).
Scheme 2.