46310-AC5
Electrokinetic Behavior and Passage of Polyelectrolytes and Surfactants throughout Tortuous Micro/Nanopore Media
The goal of this project is to better understand the potential roles of polyelectrolytes and surface-active agents in porous media for oil recovery. Our efforts will allow the formulation of smart additives to be injected into a wellbore and accomplish various objectives in a controlled way – by either promoting or resisting permeation of materials through different zones of a formation, or even in the lining adjacent to the wellbore. However, no injected polyelectrolyte or surfactant can be “smart” unless the location of injection, the type of additive, its dosage, and its time-sequence relative to other additives are properly selected. Results of the present work are part of an effort to reveal ways in which relevant additives can be used to influence permeability, depending on medium pore diameter and various controllable factors.
In the first year, we achieved significant progress towards the goal of evaluating factors affecting permeation of probe molecules into very small pores, representing those that exist within a mineral bed. The most significant milestones that were achieved so far in the project were as follows:
- Rates of penetration into the pores (nominal size 15 nm) of silica gel were strongly controlled by polymer molecular mass.
- In the case of very-low-mass polyelectrolyte the adsorption approached equilibrium very slowly, and the adsorbed amount was proportional to the square-root of time.
- With high-mass polyelectrolyte the adsorbed amount reached a pseudo-equilibrium level within about 30 minutes. These results suggest that such macromolecules permeate less than one macromolecular length into the nanopore structures.
- Changes in electrokinetic potential, resulting from progressive adsorption of cationic polyelectrolyte into nanoporous material, were found to be proportional to the logarithm of time, suggesting that at least part of the process follows Fick's law of diffusion.
- Cationic polymers having narrower molecular mass were obtained via dialysis. Confirmation of the removal of lower-mass material was obtained by using gel permeation chromatography (GPC).
- By comparing results with the dialyzed and the as-received polymer, we were able to confirm a key hypothesis to explain a reversal of streaming potential of silica gel at very high levels of polyelectrolyte treatment.. We had earlier proposed that such reversal was due to low-mass fraction in samples of high molecular weight polyelectrolytes.
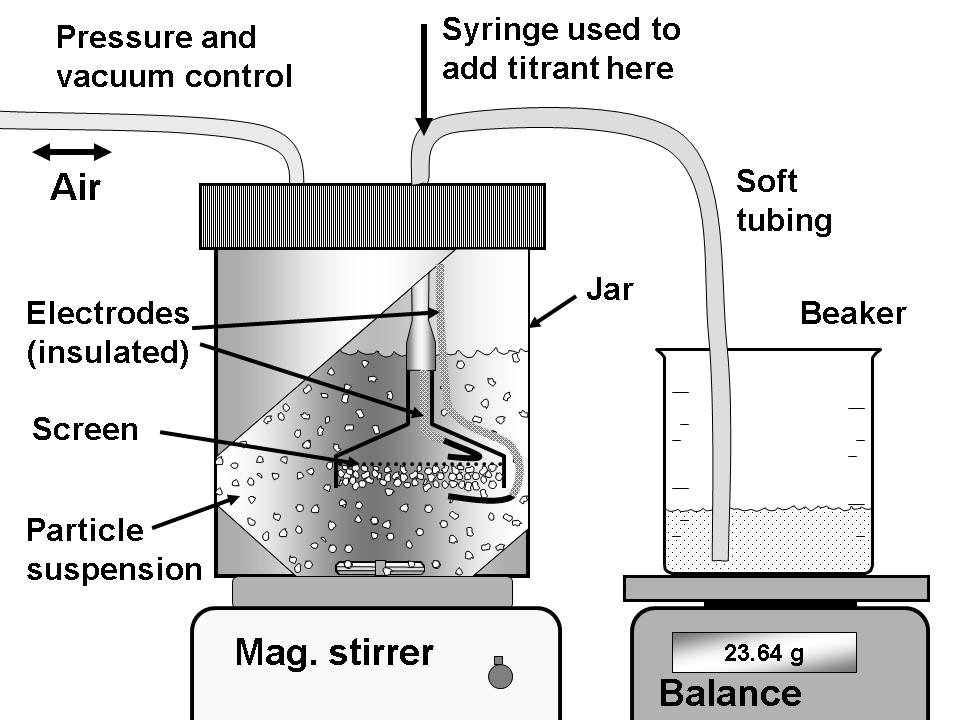
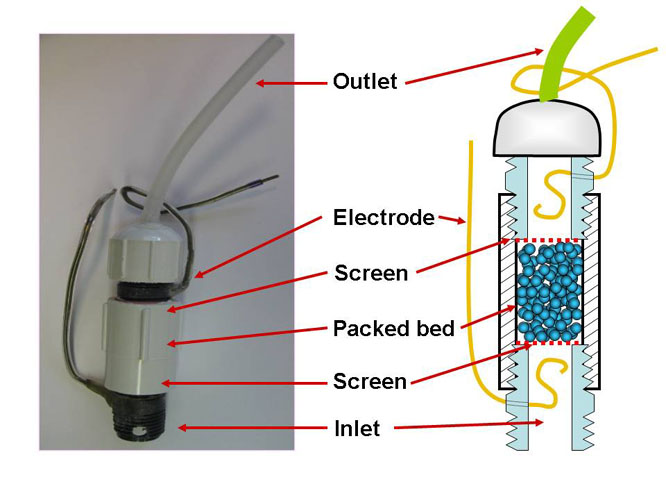
The figures shown below illustrate two versions of the streaming potential measuring device that has been developed in our laboratory and used for this project. This device, together with an innovative procedure, has made it possible to sense the passage of cationic polymers into very fine pores.
Streaming potential device using pressurized air to force a suspension toward a screen, upon which a mat of nanoporous material, such as silica gel accumulates. The electrical potential difference is measured across the mat, comparing the value with a reference value in the absense of pressure. |
Alternative measurement cell for "packed bed" experiments in which the nanoporous solid is held as a plug between fixed screens. This format makes it possible to evaluate the effects of exposing the same plug to different aqueous environments. |
A breakthrough was achieved in observing, for the first time, a trend towards more negative streaming potentials following placement of silica gel conditioned with cationic polyelectrolytes into aqueous polyelectrolyte-free solution in the presence of salt. The inferred polymer desorption is not ordinarily observed in cases where polyelectrolytes adsorb onto the outer surfaces of granular materials, but recent thermodynamic studies and simulation work have predicted such behavior in the case of pores that are smaller than the bulk-phase radius of gyration of a polyelectrolyte.
Our preliminary observations indicated that the presence of a layer of a high-mass polyelectrolyte coating the exterior surface of a nanoporous material can inhibit penetration of lower-mass probe molecules having the same electrical charge. Understanding and controlling such phenomena can help to influence the progress of different solutes permeating through different zones of an oilfield operation.
Current efforts are being placed in two areas, namely, factors affecting the diffusion of probe materials into pore spaces, and verification of permeation data. Independent variables of interest are salt concentration (to control the Debye-Hückel parameter governing electrostatic interactions), pH (to control the charge of the silica gel substrate), the pore size (by selecting different silica gel samples), and the use of surfactants (to test a hypothesis that such additives can act as “carriers” for other materials, helping them to progress throughout a fine-porous substrate). To provide verification of our novel electrokinetic test procedures, we are working up optical tests involving confocal laser scanning microscopy, in conjunction with tagged DADMAC copolymers.