ACS PRF | ACS
All e-Annual Reports

43861-G6
Helix Macro-Dipolar Effects on the Acidities of Cysteine-Containing Helical Peptides
The goal of this research is to develop a quantitative model of the helix macro-dipolar effects on the acidities of the cysteine-containing peptides. During the first funding year, we focused on method development by using two series of cysteine-polyalanine peptides, one with the cysteine residue at the N-termini, Cys-(Ala)n-NH2 (HSCAn), and the other with the cysteine residue at the C-termini, (Ala)n-Cys-NH2 (AnCSH), where n = 3-9. The peptides were synthesized in our laboratory via solid phase peptide synthesis. The gas-phase acidities were determined by performing mass spectrometry measurements. The conformations of the peptides were modeled via quantum chemical calculations as well as molecular dynamics simulations. One paper on this subject has been published and one manuscript has been submitted for publication. The results have also been presented in several national and international conferences.
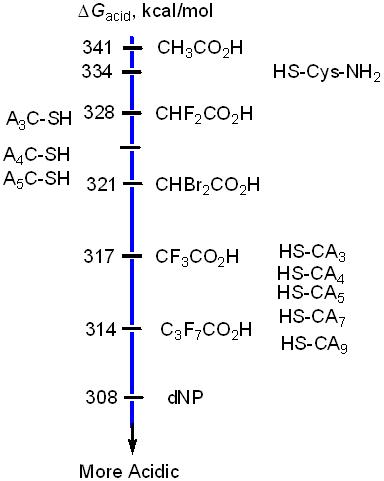
For the purpose of establishing the mass spectrometry method, we first studied two smaller peptides with the general formula, Cys-( The same mass spectrometry method was applied to the longer N-cysteine peptides, Cys-( We also studied several smaller C-cysteine peptides, ( We modeled the conformations of the smaller peptides with three to six amino acid residues using semiempirical (AM1) and the quantum chemical (DFT) methods. The results show that the deprotonated N-cysteine peptides exist as partial helices, while the deprotonated C-cysteine peptides exist as random coil. The differences in the conformations may be responsible for the differences in the acidities of the small C-cysteine peptides as compared to the N-cysteine ones. In collaboration with Dr. C. Michael McCallum (University of the Pacific), we also performed molecular dynamics simulations on selected longer peptides with amino acid residues greater than eight. The results agree with our predictions that the neutral and deprotonated N-cysteine peptides exist in helices in the gas-phase, while the deprotonated C-cysteine peptides adopt globular conformations. Future work will involve: 1) Modification of the current experimental method, so that the acidities of the longer peptides could be measured more accurately. 2) Investigate peptide sequence effects on the acidity of the cysteine residue when the cysteine is placed in the different positions of the peptide chain. The sequence effects are related to the conformational effects. 3) Expend the peptides to the other cysteine-containing peptides, such as, cysteine-polyglycine peptides. The results from these studies should produce sufficient data to correlate the lengths of the peptides to the gas-phase acidity enhancements in the N-cysteine helical peptides. The lengths of the peptides are directed related to the magnitude of the helix macro-dipole moments. Finally, computational studies will be carried out to examine solvent effects on the acidity enhancements of the helical peptides. Several undergraduates as well as one graduate student have gained significant research experience while participating in this research. This research has led to a continuing project for undergraduate students.