
ACS PRF | ACS
All e-Annual Reports

43048-AC3
Activation of Nitric Oxide at Low-Coordinate Fe, Co, Ni, and Cu Centers
Nitric oxide (NO) is an undesirable product of high-temperature hydrocarbon combustion, reacting swiftly with oxygen in the air to form nitrogen dioxide (NO2), a brown gas which is a principle component of smog, irritating to mucous membranes. With the introduction of automobile catalytic converters, NOx emissions have dropped by 70 – 90 %. These three way catalysts consist of oxide supported late transition metal atoms which both bind NO as well as assist its disproportionation to surface-bound N and O atoms which recombine to give the thermodynamically favored N2 and O2. Nonetheless, the traditional three-way catalysts based on Pt and Rh are not effective in the O2-rich exhaust of diesel and lean-burn engines, motivating a new zeolite-based catalysts incorporating less expensive first-row metals.
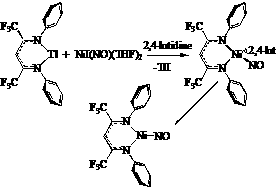 During the second year of this two-year PRF grant, we have explored the ligand variations in three-coordinate nickel nitrosyl complexes to understand their effect on the coordinated nitrosyl ligand. Addition of the thallium b-diketiminate Tl[H2NNF] to NiI(NO)(THF)2 in the presence of 2,4-lutidine results in the structurally characterized tetrahedral [H2NNF]Ni(NO)(2,4-lutidine) which binds lutidine rather weakly in solution. The use of BF3•OEt2 cleanly removes the substituted pyridine to afford the three-coordinate [H2NNF]Ni(NO). The electron-withdrawing nature of the fluorinated b-diketiminate ligand results in a significantly increased nNO stretching frequency of 1827 cm-1.
During the second year of this two-year PRF grant, we have explored the ligand variations in three-coordinate nickel nitrosyl complexes to understand their effect on the coordinated nitrosyl ligand. Addition of the thallium b-diketiminate Tl[H2NNF] to NiI(NO)(THF)2 in the presence of 2,4-lutidine results in the structurally characterized tetrahedral [H2NNF]Ni(NO)(2,4-lutidine) which binds lutidine rather weakly in solution. The use of BF3•OEt2 cleanly removes the substituted pyridine to afford the three-coordinate [H2NNF]Ni(NO). The electron-withdrawing nature of the fluorinated b-diketiminate ligand results in a significantly increased nNO stretching frequency of 1827 cm-1. 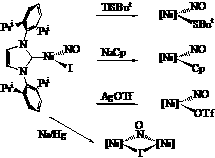 We have also begun to explore the use of monodentate supporting ligands to achieve low-coordinate metal nitrosyls. The addition of a free N-heterocyclic carbene (NHC) to NiI(NO)(THF)2 results in the formation of the three-coordinate [NHC]Ni(I)(NO) which exhibits a nNO stretch at 1766 cm-1. This is amenable to further functionalization via reactions with a variety of nucleophiles X- to deliver [NHC]Ni(X)(NO) species (SBut, with nNO = 1750 cm-1 or Cp with nNO = 1810 cm-1). Moreover, [NHC]Ni(I)NO undergoes reduction with sodium amalgam to give the dinuclear species {[NHC]Ni}2(m-I)(m-NO) (vNO = 1683 cm-1) with a very short Ni-Ni distance of 2.297 Ǻ)
We have also begun to explore the use of monodentate supporting ligands to achieve low-coordinate metal nitrosyls. The addition of a free N-heterocyclic carbene (NHC) to NiI(NO)(THF)2 results in the formation of the three-coordinate [NHC]Ni(I)(NO) which exhibits a nNO stretch at 1766 cm-1. This is amenable to further functionalization via reactions with a variety of nucleophiles X- to deliver [NHC]Ni(X)(NO) species (SBut, with nNO = 1750 cm-1 or Cp with nNO = 1810 cm-1). Moreover, [NHC]Ni(I)NO undergoes reduction with sodium amalgam to give the dinuclear species {[NHC]Ni}2(m-I)(m-NO) (vNO = 1683 cm-1) with a very short Ni-Ni distance of 2.297 Ǻ) 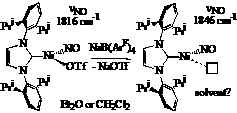 Towards the goal of generating isolable, unprecedented two-coordinate transition metal-nitrosyls, we performed an anion exchange of the iodide in [NHC]NiI(NO) with AgOTf to provide [NHC]Ni(OTf)NO. This possesses a somewhat T-shaped geometry (C-Ni-N = 121.59˚, C-Ni-O = 101.06˚, N-Ni-O = 137.27˚) that could be reproduced by high-level DFT calculations on the full molecule (C-Ni-N = 123.2˚, C-Ni-O = 101.7˚, N-Ni-O = 135.0˚). Its nNO stretching frequency of 1826 cm-1 is significantly higher than that of the iodide, reflecting the poorer donating ability of the triflate. Reaction with NaBArF4 (ArF = 3,5-(CF3)2C6H3 in either ether or CH2Cl2 results in identical NMR spectra in CDCl3. Moreover, solution IR spectra of this putative cationic species in either ether or CH2Cl2 reveal one nNO of 1846 cm-1, suggesting that neither solvent coordinates to the metal, or they coordinate in a very similar manner. Further spectroscopic and isolation attempts continue in an effort to better understand this curious, extremely low-coordinate species.
Towards the goal of generating isolable, unprecedented two-coordinate transition metal-nitrosyls, we performed an anion exchange of the iodide in [NHC]NiI(NO) with AgOTf to provide [NHC]Ni(OTf)NO. This possesses a somewhat T-shaped geometry (C-Ni-N = 121.59˚, C-Ni-O = 101.06˚, N-Ni-O = 137.27˚) that could be reproduced by high-level DFT calculations on the full molecule (C-Ni-N = 123.2˚, C-Ni-O = 101.7˚, N-Ni-O = 135.0˚). Its nNO stretching frequency of 1826 cm-1 is significantly higher than that of the iodide, reflecting the poorer donating ability of the triflate. Reaction with NaBArF4 (ArF = 3,5-(CF3)2C6H3 in either ether or CH2Cl2 results in identical NMR spectra in CDCl3. Moreover, solution IR spectra of this putative cationic species in either ether or CH2Cl2 reveal one nNO of 1846 cm-1, suggesting that neither solvent coordinates to the metal, or they coordinate in a very similar manner. Further spectroscopic and isolation attempts continue in an effort to better understand this curious, extremely low-coordinate species. To identify the reactivity of NO and NO2 in the absence of a redox-active metal center, we utilized zinc tris(pyrazolyl)borate complexes. While the zinc-thiolates iPr2TpZn-SBut are stable towards free NO, they react instantaneously with NO2 (or aerobic NO) to give tBuSNO and iPr2TpZn(NO3). The observation of the zinc-nitrate indicates that NO2 (N2O4 ≡ [NO]+[NO3]-) is the active nitrosating agent. Moreover, transnitrosation with S-nitrosothiols RSNO readily takes place at iPr2TpZn-SR' complexes in CDCl3 to provide equilibrium mixtures of R'SNO and iPr2TpZn-SR. A second-order rate constant of 2.0(0) M-1 s-1 was obtained at 60 °C for the degenerate exchange between PhCH2SNO and iPr2TpZn-SCH2Ph. Further work with copper is in progress, especially given the success of the copper-exchanged zeolite Cu-ZSM-5 to serve as a photocatalyst for NO decomposition as well as the synthetic accessibility of TpCu(NO) complexes.
To identify the reactivity of NO and NO2 in the absence of a redox-active metal center, we utilized zinc tris(pyrazolyl)borate complexes. While the zinc-thiolates iPr2TpZn-SBut are stable towards free NO, they react instantaneously with NO2 (or aerobic NO) to give tBuSNO and iPr2TpZn(NO3). The observation of the zinc-nitrate indicates that NO2 (N2O4 ≡ [NO]+[NO3]-) is the active nitrosating agent. Moreover, transnitrosation with S-nitrosothiols RSNO readily takes place at iPr2TpZn-SR' complexes in CDCl3 to provide equilibrium mixtures of R'SNO and iPr2TpZn-SR. A second-order rate constant of 2.0(0) M-1 s-1 was obtained at 60 °C for the degenerate exchange between PhCH2SNO and iPr2TpZn-SCH2Ph. Further work with copper is in progress, especially given the success of the copper-exchanged zeolite Cu-ZSM-5 to serve as a photocatalyst for NO decomposition as well as the synthetic accessibility of TpCu(NO) complexes.