
ACS PRF | ACS
All e-Annual Reports

44760-B6
Thermodynamics and Kinetic Studies of Hydrogen Isotope Binding on Selective Materials
 As a first step toward understanding the thermodynamics and kinetics of tritium exchange in a variety of materials, we considered the tritiated water auto-disssociation equilibria.
As a first step toward understanding the thermodynamics and kinetics of tritium exchange in a variety of materials, we considered the tritiated water auto-disssociation equilibria. 
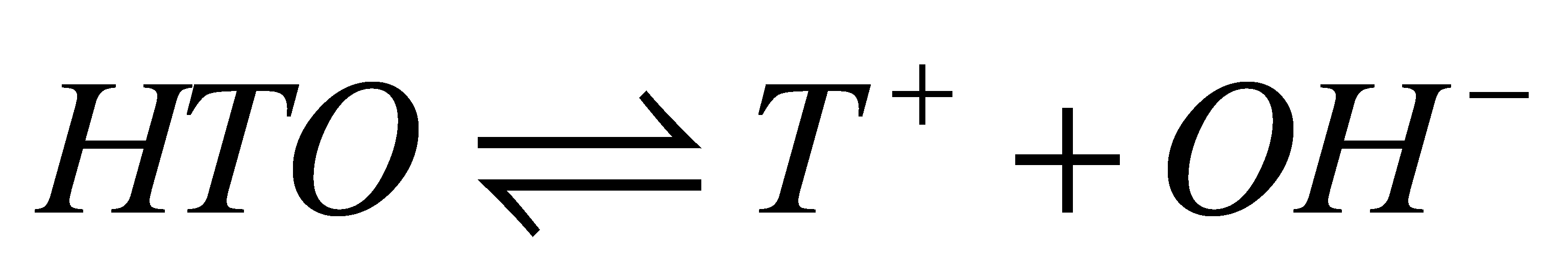
Identifying the preferred form of the tritium is key to choosing materials that can exchange it. To find the change in free energy of each of these reactions, we found the solvation free energy of the individual species using a quasi-chemical theory of solvation.1-4 Lindiwe Ndebele (2009) and Laura Fernandez (2008) have found the most probable solvated structures for T+ and TO- using a quasi-chemical theory of solvation with harmonic and anharmonic frequency estimates. The most probable species using harmonic frequency estimates are the same as those found in previous quasi-chemical applications on H+ and HO-.1-4
Since differences between isotopes are crucial for the auto-dissociation of tritiated water, we have considered anharmonic frequencies. A second order pertubation approach yielded highly suspect frequencies for these highly anharmonic systems. We have also considered simple scaling relations. With scaled harmonic frequencies, we find a pKw of 15 rather than 14 for H2O and both HTO dissociations at room temperature. Since experimental studies suggest that raising the temperature from 20°C to 60°C changes the preferred HTO dissociation, we are considering higher and lower temperatures.
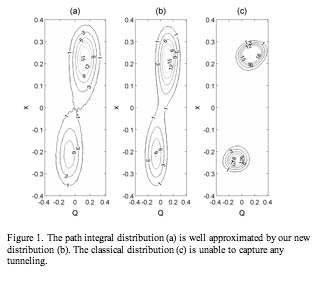
In addition, we starting to use the MULTIMODE method of Bowman and Carter. This method not only yields more accurate anharmonic energies but also finds wave functions for the most important solvated species. In addition to using the anharmonic frequencies in our study of the HTO dissociation, the wave functions will be used within our new quantum system/classical bath approximation of the density matrix to yield a quantum/classical distribution for Monte Carlo simulations. The resulting distribution approximated the path integral distribution very well for a simplified system as shown in Figure 1.5
1. L. R. Pratt, R. A. LaViolette, M. A. Gomez, and M. E. Gentile*, "Quasi-chemical Theory for the Statistical Thermodynamics of the Hard Sphere Fluid." J. Phys. Chem. B. 105, 11662 (2001).
2. P. Grabowski*, D. Riccardi*, M. A. Gomez, D. Asthagiri, and L. R. Pratt, "Quasi-chemical theory and the standard free energy of H+ (aq)," J. Phys. Chem. A. 106, 9145 (2002).
3. D. Asthagiri, L. R. Pratt, J. D. Kress, and M. A. Gomez, "The hydration state of HO-," Chemical Physics Letters 380, 530 (2003).
4. D. Asthagiri, L. R. Pratt, J. D. Kress, and M. A. Gomez, "Hydration and mobility of HO- (aq)," Proc. Natl. Acad. Sci. USA 101, 7229 (2004).
5. M. A. Gomez, and P. Peart*, "Including quantum subsystem character within classical equilibrium simulations," J. Chem. Phys. 125, 034105 (2006).