
ACS PRF | ACS
All e-Annual Reports

45505-GB3
Development of Transition Metal Catalysts for Environmentally Benign Oxidation of Organic Compounds by Nitrous Oxide
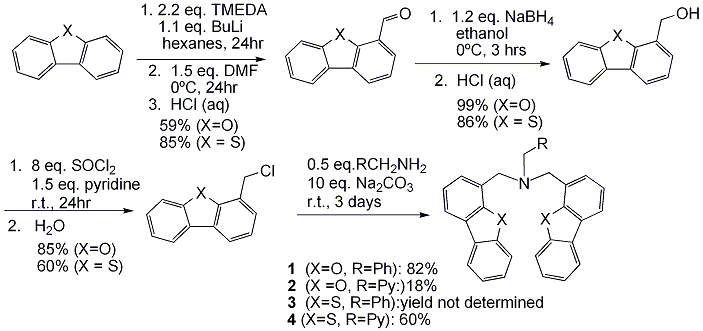
We have synthesized new multidentate ligands with dibenzofuranyl, dibenzothiophenyl and indolyl groups as bulky donor groups in order to develop homogeneous transition metal catalysts for “green” oxidation of hydrocarbons. The tridentate and tetradentate dibenzofranyl ligands 1 and 2 have been synthesized in four steps. Most of previously reported dibenzofuranyl-group-containing ligands had dibenzofuran as a rigid backbone, non-coordinating to the metal center. Ligands 1 and 2 were created to use the dibenzofuranyl group as an active donor group. The preliminary metal complexation studies with the tridentate ligand 1 showed that the ligand does not react with Fe(NCCH3)2(OTf)2 or [Cu(NCCH3)4]]PF6 but quantitatively reacts with TiCl4 and VOCl3. The full characterization of these early transition metal adducts is currently in progress. Ligand 2, the tetradentate analogue, did react with all the four metal sources mentioned above, showing that the fourth pyridine moiety helps binding to late transition metals Fe2+ and Cu+ as well. Similar reactivity was observed with the sulfur analogues 3 and 4, showing little difference between the O and S donor atoms in the same framework.
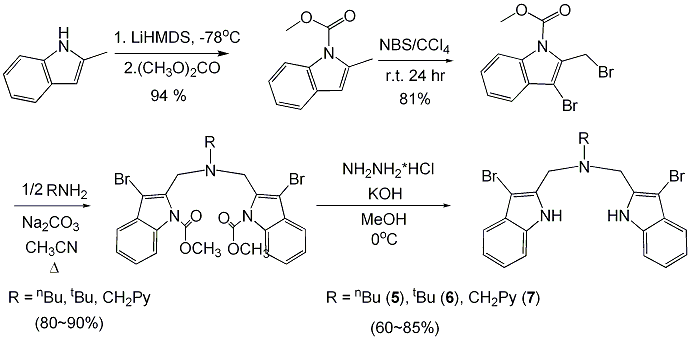
Three indole-group-containing ligands (5, 6, 7) have been synthesized in five steps (with the common first three steps). The 3-position of the indolyl group was brominated so that later this position can be functionalized with either electron-withdrawing or donating groups. The indolyl groups in ligands 5, 6, and 7 will be deprotonated with a strong base before metal complexation in order to provide negatively charged bulky donor groups. The complex of the deprotonated ligand with a M2+ ion will be charge-neutral, thus would be soluble in organic solvents. The metal complexation with these ligands has been challenging, and we are still in the process of finding suitable combinations of base-solvent-metal source for clean reactions.
Our future work will involve the full characterization of the metal complexes and testing them as catalysts for oxidation of hydrocarbons with environmentally benign oxidants such as N2O and H2O2. Other O-atom transfer agents such as peroxides, iodosobenzene and hypochlorites will be studied as well. Syntheses of other ligands have been initiated and we will aim to complete the syntheses within the next year.
In the past year (September 2006 to August 2007) the grant allowed a significant advance of the project and provided vigorous research experiences for six undergraduate students. The grant paid for chemicals, supplies, and elemental analysis throughout the year that enabled us to complete the synthesis of seven new ligands and to explore metal complex formations. The grant financially supported three summer students and the principal investigator between May and August 2007. During the fall and spring semesters (August 2006 to May 2007) five undergraduate students actively contributed to the project. We are currently refining the metal complexation processes and are aiming to write two manuscripts for publication by spring 2008. All six undergraduate students have been trained on handling air sensitive compounds, flash column chromatography, the use of a glove box, and routine acquisition of 1H and 13C NMR spectra. All of the students presented or co-presented a poster at national and/or regional ACS meetings. A recent graduate, Ashley Ribera, accepted a research position at the Center for Disease Control and Prevention.