ACS PRF | ACS
All e-Annual Reports

42227-AC9
Exploring the Effects of Self and Cross-Clustering on the Thermodynamics of Strongly Associating Systems
The goal of this research was to develop a robust and predictive thermodynamic model for the aqueous-HF system through the use of parameter fitting to experimental data as well as the use of ab initio techniques and molecular simulation to refine association schemes while providing additional information. The thought behind this is that if we could integrate all of this information together in a successful way, then this approach could be modified for other, highly-associating systems.
DFT Cluster Studies for the Aqueous Hydrogen Fluoride System
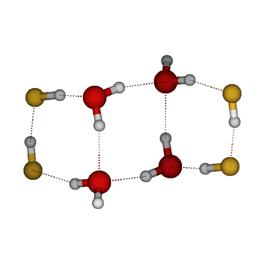
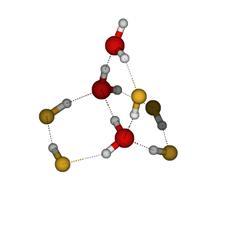
In order to identify the cross-association patterns that are likely to be found in the solution, the distribution of association patterns in (HF)m(H2O)n clusters were investigated using cluster studies. This study was performed at the mPW1B95/6-31+G(d,p) level of theory. A total of 214 optimized geometries of (HF)m(H2O)n clusters, with m + n as high as 8, were investigated. The strongest H-bond interaction was reported to be the H2O…H–F interaction. It was also found that the larger (HF)m(H2O)n clusters and the clusters with equimolar contributions of HF and H2O were preferred in the solution phase. Some sample clusters are provided in Figure 1.
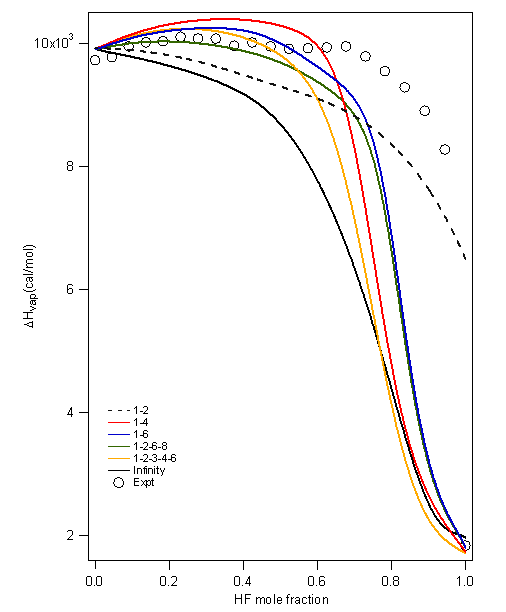
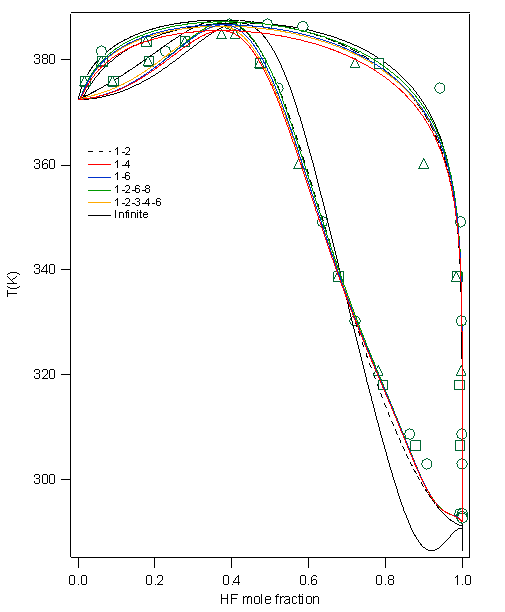
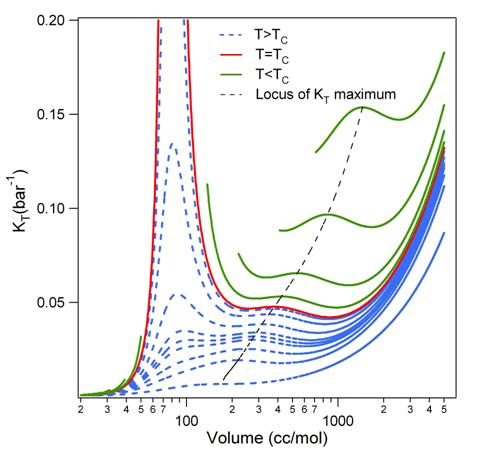
Figure 1: The figure on the left shows an 8-mer of equal composition forming a chair arrangement. The figure on the right shows a bridge for an 8-mer formed by five HF molecules and three water molecules. Aqueous-HF Mixtures: Thermodynamic Models We have explored fourteen HF models which comprise a variety of association schemes. These models have been optimized through parameter fitting for the two-phase system and then tested versus various experimental data not used during parameterization. We explore the predictive ability of models for mixtures where only self-association is included. These are provided in Figure 2 for the bubble and dew point curves at atmospheric pressure. Figure 3 shows the prediction of heat effects for some self-association mixture models Figure 2: A Txy diagram at 1 atm for the aqueous HF system for a variety of self-association models for hydrogen fluoride. Figure 3: Prediction of the heat of vaporization for the mixture as a function of mole fraction from a variety of models. All perform poorly in the concentrated-HF region. We have developed proper approaches and written codes to incorporate cross-association into the thermodynamic models. This is being assessed through binary interaction parameters. The less important this parameter is, the more predictive the model. Work is on-going to identify the best models through this approach. Knowledge of the DFT clustering studies has informed the cross-association models. Results are provided in Figure 4. Here, only dimer cross-association has been included. Figure 4: A Txy diagram at 1 atm for the aqueous HF system for a variety of self-association and cross-association models for hydrogen fluoride. Isothermal Compressibility Maximum for Pure HF While in conference with a colleague on HF modeling, we have uncovered that some of our pure component models for HF will predict a second isothermal compressibility (KT) maximum in the super critical region. When the system is decompressed, this additional peak extends well into the super heated vapor region. Figure 2 shows the KT behavior from one of the models. To our knowledge there is no modeling or experimental work reporting a KT peak in the super heated vapor region for any substance. We believe that this is due, once again, to the marked change from a highly-associated state to a non-associated state in the vapor phase of HF. Figure 5: KT results for HF. The model predicts the critical temperature to be 473.1 K Note that the black dashed line, which shows that locus of second-maxima, ends at 668 K. Analytical 1-2-3 Model While working on the correlation and prediction of pure HF properties from several association models, we obtained an analytical closed-form (exact) expression for an association scheme that allows the formation of monomers, dimers and trimers only. To demonstrate the utility of this exact solution, the phase equilibrium results of acetic acid were obtained from this exact expression and were compared with a numerical approach as well as a fitted functional form. The exact form was preferred over the numerical as well as the fitted function form based on issues that arise from the computational time (the former) and accuracy of derivative properties (the latter). Funding Impact Funding this research has made a large impact on the graduate student supported by the PRF. By November of 2007, he will have given five presentations at the Annual AIChE meeting disseminating the results of this research and is co-author on two papers pertaining to this work, with more to follow. Additionally, his improved knowledge of ab initio techniques, pure component parameterization and thermodynamics, in general, speaks to the impact that this funding has provided. He has graduated with his Ph. D. and has taken an industrial position.