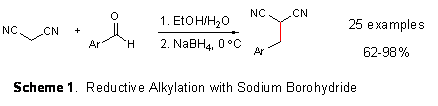ACS PRF | ACS
All e-Annual Reports

42848-GB1
Borohydrides as Reagent and Catalyst in Carbon-Carbon Bond Formation
From previously published research in my group (Synthesis 2006, 680-686), investigations for the grant continued this past year. The novel reductive alkylation reaction with sodium borohydride was examined for the synthesis of monosubstituted malononitriles by utilizing aromatic aldehydes as shown in Scheme 1 (carbon-carbon bond formation shown in red). There were issues with scope and using only aqueous ethanol for the condensation step solved them. Research is continuing to address these issues and increase the impact of the reaction as shown below.
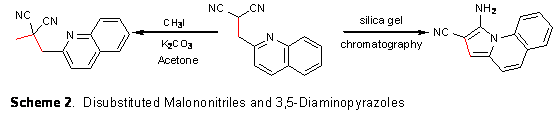
2-Quinolinecarboxaldehyde produces a monosubstituted malononitrile from the carbon-carbon bond forming reductive alkylation, but upon attempted recrystallization or column chromatography a rearrangement occurs. Nucleophilic attack of the quinoline nitrogen on the carbon of the nitrile followed by a proton transfer and a tautomerization explain how the indolizine is created (Scheme 2). Single crystal x-ray analysis was applied to confirm the structure of this novel rearranged product. Under alkylation conditions, only C-alkylation occurs to form a disubstituted malononitrile, which does not undergo rearrangement. Based on the selectivity for C-alkylation (verses N-alkylation) on the above quinoline derivative we have explored the reaction of the pyridyl derivatives (Scheme 3). Additional alkylating agent subsequently allows for N-alkylation. We are currently now examining the reaction of phenol containing malononitriles. When salicylaldehydes (2-hydroxybenzaldehydes) are used the reaction takes a different course where condensation and cyclization precede reduction and new heterocycles are prepared. This one-pot reaction to produce 4H-chromenes is shown in Scheme 4. The scope and limitations of this reaction as well as the utilization of the products are still being addressed. We have discovered that if we allow the reductive alkylation to return to room temperature and stir for 24 hours that one of the nitriles is converted to an ethyl ester. This one-pot reaction is shown in Scheme 5. The scope of this reaction and utilization of the products are still being addressed. The resources provided by this ACS-PRF grant have helped my career get off to a swift start. There are several important areas of impact from this funded research. One is the impact to the chemistry community from the journal publication and numerous oral and poster presentations at local, regional and national meetings. I was an author of four presentations/posters at the national ACS meeting in