
ACS PRF | ACS
All e-Annual Reports

42312-G3
Bridging Biology and Nanochemistry: Metalloclusters as Bioimaging Agents
and Biomineralization Scaffolds
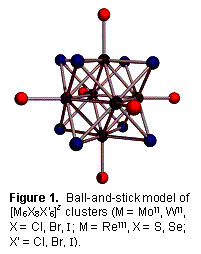 A principal research focus in this laboratory since inception has been the
synthesis and photochemical characterization of metal-metal bonded clusters. Of primary interest are the hexanuclear
molybdenum(II) and tungsten(II) halide clusters and the chalcogenide clusters
of rhenium(III). Figure 1 depicts their
structure. An octahedron of metal atoms
resides within a cube of face-bridging ligands: halides for MoII and
WII; sulfide or selenide for ReIII. Apical capping ligands radiate outward from
the metallocore. These entities
collectively constitute the largest series of isoelectronic metal-metal bonded
clusters known. Undoubtedly their
leading feature is their luminescence.
Emission results from excitation with ultraviolet or blue light;
luminescence quantum yields in solution range from ca. 125% at room
temperature, and triplet-state lifetimes are on the order of microseconds.
A principal research focus in this laboratory since inception has been the
synthesis and photochemical characterization of metal-metal bonded clusters. Of primary interest are the hexanuclear
molybdenum(II) and tungsten(II) halide clusters and the chalcogenide clusters
of rhenium(III). Figure 1 depicts their
structure. An octahedron of metal atoms
resides within a cube of face-bridging ligands: halides for MoII and
WII; sulfide or selenide for ReIII. Apical capping ligands radiate outward from
the metallocore. These entities
collectively constitute the largest series of isoelectronic metal-metal bonded
clusters known. Undoubtedly their
leading feature is their luminescence.
Emission results from excitation with ultraviolet or blue light;
luminescence quantum yields in solution range from ca. 125% at room
temperature, and triplet-state lifetimes are on the order of microseconds.
The principle investigator works to apply the excited-state properties of these clusters in biological settings, for optical biological imaging and photodynamic therapy. Present efforts seek (a) amelioration of the toxicity associated with their heavy-metal compositions and (b) optimization of their multiphoton absorption capabilities.
Multiphoton-absorbing Metalloclusters. Toward Reagents for Two-Photon Photo-dynamic Therapy.
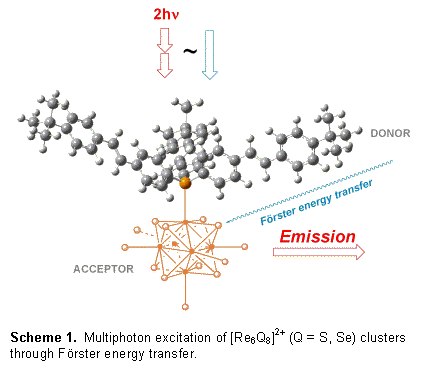
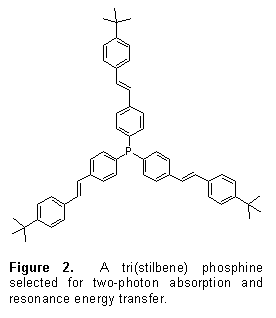
Two-photon excitation, when combined with photodynamic therapy allows ultraprecise cancer treatment in sensitive anatomical regions, such as the brain. The Gray research laboratory has devised an energy transfer scheme, where multiphoton-harvesting antennae funnel energy into the triplet states of metalloclusters. Scheme 1 depicts the excited-state concept. Multiphoton excitation of the pendant stilbene moieties leads promptly to a ligand-centered singlet state. Fφrster energy transfer to the cluster, combined with intersystem crossing, affords a long-lived (microseconds) cluster-centered triplet state. This triplet excited state then sensitizes oxygen to form therapeutic 1O2. For these initial investigations, tri(stilbene) phosphine 1 of Protasiewicz and co-workers[1] was chosen as a two-photon absorber, Figure 2. Stilbenes and their derivatives are now established two-photon chromophores, and tri(stilbene) phosphines are available from commercial reagents in gram quantities.
Scheme 2 depicts a general strategy for ligand attachment to cluster cores. Refluxing an N,N-dimethylformamide solution of the halide-terminated clusters with the free ligand affords site differentiated species [Re6Se8(Pstil3)nI6n]2n (a). Clusters bearing four or five tri(stilbene) phosphines are obtainable this way.
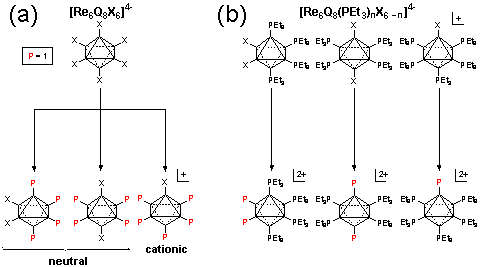
Clusters having a smaller loading of tri(stilbene) phosphine ligands are obtainable from species passivated beforehand with an inert phosphine. A family of [Re6Q8(PEt3)nX6 n]2 n (Q = S, X = Br; Q = Se, X = I) clusters is available from previous work.[2],[3] In these species, the remaining halides are replaceable, either by direct substitution with phosphine or by de-halogenation with AgI or TlI reagents, Scheme 2 (b). Efforts to obtain mixed phosphine clusters are underway. Two-photon absorption cross section measurements are pending in the laboratory of Professor D. G. Nocera, MIT. Results of these investigations will be disclosed with due speed.