ACS PRF | ACS
All e-Annual Reports

43745-AC4
Hydrocarbon, Fluorocarbon, and Mixed Hydrocarbon/Fluorocarbon Liquid Crystalline Materials from Predictable Supramolecular Assembly of Piperazine-2,5-Diones
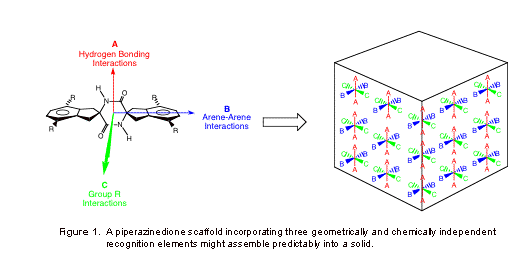
Our program in crystal engineering (CE) seeks to harness intermolecular interactions for the design and production of novel molecules that exhibit useful bulk properties. We are employing a piperazinedione-based molecular scaffold (see Figure 1) to explore the effects of the aryl appendages R on the crystal packing and bulk properties of such molecules. Our working hypothesis has been the crystal packing of symmetrically substituted tetra-n-alkoxypiperazinediones 1 will be governed by (1) assembly of these molecules into "one-dimensional" tapes through establishment of reciprocal amide hydrogen bonds, (2) assembly of the tapes into "two-dimensional" grooved sheets through establishment of edge-to-face arene interactions, and (3) assembly of the sheets into "three-dimensional" solids by "tongue-in-groove" interdigitation of the n-alkyl "tails" in extended conformations, independent of chain length. Liquid crystal (LC) properties would be expected for compounds bearing alkyl groups of sufficient length. We proposed to complete the study of tetra-n-alkoxypiperazinediones 1 and to expand the study to include symmetrically substituted tetra-n-perfluoroalkylpiperazinediones 2 and hybrid hydrocarbon/fluorocarbon piperazinediones 3 and 4 with a goal of developing novel fluorocarbon and mixed hydrocarbon/fluorocarbon liquid crystalline materials.
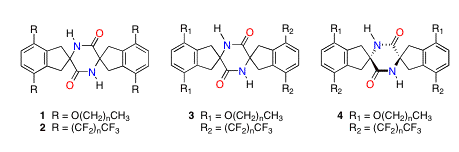
We have completed the syntheses of the series of compounds 1 with n = 1, 2, 4, 6, 8, 9, and 12. Crystallography for the compounds 1 with n = 1, 2, 4, and 12 has been completed. Crystals of the compounds 1 where n = 6, 8, and 9 were sent in September 2007 to Service Crystallography at the Advanced Light Source (SCrALS) for data collection using synchrotron radiation. This was necessary due to the small size of the crystals. It is hoped that the synchrotron data sets will lead to solved crystal structures. The thermochemical properties of molecules 1 have been studied, and they do exhibit LC phase transitions as expected. Submission of a full paper disclosing our results with compounds 1 is planned for later this year.
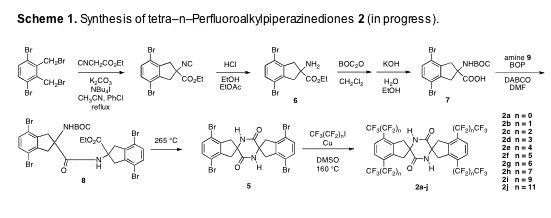
Progress has also been made toward the syntheses of tetra-n-perfluoroalkylpiperazinediones 2. Our synthesis of these compounds is depicted in Scheme 1. Tetrabromide 5 has been prepared from known a,a',3,6-tetrabromo-o-xylene via coupling of amino ester 6 with BOC acid 7 and thermally induced cyclization of the resulting dipeptide 8. Copper mediated coupling of tetrabromide 5 with appropriate commercially available perfluoroalkyl iodides should provide 2a-j.