ACS PRF | ACS
All e-Annual Reports

42910-AC3
Probing Reactivity and Coordination Limits of Open Geodesic Polyaromatic Hydrocarbons in Metal Binding Reactions
This project is focused at the exploration of the reactivity and coordination limits of a new class of hydrocarbons, namely open geodesic polyarenes (buckybowls), in which inside and outside carbon surfaces exhibit different properties. An original and effective gas phase deposition approach has been used to test the coordination preferences of buckybowls in metal binding reactions. These investigations explore fundamental metal-π-arene interactions with an emphasis on the perturbation of structure and reactivity induced by metal coordination to non-planar carbon-rich polyaromatic surfaces.
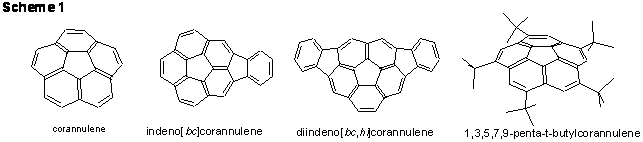
Several computational methods used for calculating molecular geometries of buckybowls have been evaluated and calibrated against single crystal X-ray diffraction data. The reliability and shortcomings of geometry calculations at several levels of theory have been enumerated using a good quality X-ray data set for the corannulene bowl obtained in our group. This was followed by the first X-ray structural characterization of new bowl-shaped polyarenes: 1,3,5,7,9-penta-tert-butylcorannulene, monoindeno- and diindenocorannulenes (Scheme 1). These structural studies revealing geometry and solid state packing of bowl-shaped molecules were accompanied by evaluation of their geometries and electronic structures using DFT-methods (DFT/PBE0). It was found that the addition of indeno groups imposes additional curvature to the corannulene core which should affect the hydrocarbon reactivity. In contrast, adding bulky tert-butyl groups to the rims of corannulene flattens the bowl but also alters the ligating properties of the polyarene. Thus, while multiple metal coordination of electrophilic rhodium(II) centers of dirhodium(II,II) tetra(trifluoroacetate) units to the rim sites of the corannulene bowl has been readily achieved under gas phase reaction conditions, our attempts to coordinate metals to 1,3,5,7,9-penta-tert-butyl-corannulene have been unsuccessful. The presence of bulky substituents hinders access of the metals to the reactive rim sites of the bowl, as anticipated. However, this modification of the corannulene core has not fulfilled our hope of diverting complexation to the unsaturated bowl surface. This goal, however, was achieved by controlled tuning the electrophilicity of metal centers utilized for buckybowl coordination. Thus, the use of mixed carbonyl carboxylate complex of ruthenium(I), which is a much softer Lewis acid than dirhodium(II,II) tetra(trifluoroacetate), has resulted in the hub-coordinated complex of corannulene. This was a unique example that demonstrated for the first time a degree of similarity between the ligating properties of the convex carbon surface of corannulene and that of the C60-fullerene.
This observation along with the continued absence of any other experimental examples confirming ‘fullerene-like” properties of bowl-shaped polyarenes, prompted us to directly compare the coordination abilities of corannulene vs. C60-fullerene. As a result of theoretical modeling of binding the electrophilic ruthenium(I) center to the same carbon-carbon site at the convex unsaturated carbon surfaces of C20H10 and C60, a significant difference of their donor-acceptor properties has been revealed and quantitatively estimated for the first time. While the donating properties of corannulene and the fullerene are confirmed to be close, the fullerene ligand appeared to be a much better acceptor in metal binding reactions. In the course of the above research activities, a program was created for training young chemists in syntheses, crystal growth, various characterization techniques, and X-ray diffraction. One postdoctoral associate, three graduate and five (one minority and four female) undergraduate students participated in this program that facilitated their professional development and prepared students for the next career step. Four participating undergraduates plan to continue their education in chemistry at the graduate level.