ACS PRF | ACS
All e-Annual Reports

41181-B3
Influence of Octahedral Coordination Geometry on Catalyst Performance
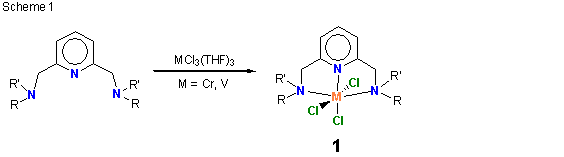
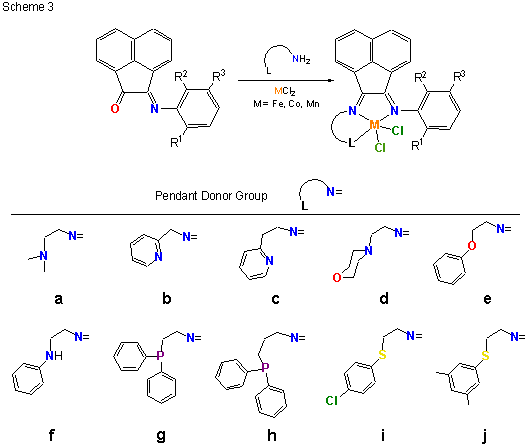
During this reporting period, we continued to examine the structure and ethylene polymerization performance of transition metal complexes supported by tridentate, mer-coordinating ligands. Most of our effort focused on chromium, vanadium and iron-containing compounds with the goal of correlating catalyst performance with metal type and ligand structure. Toward this end, chromium and vanadium complexes supported by ligands with 2,6-disubstituted pyridine (structure 1 in Scheme 1) and pyridine-2-carboxaldehyde (structure 2 in Scheme 2) backbone structures were prepared and characterized. Several examples of 1 and 2 (M = Cr, V) were structurally characterized by x-ray crystallography and all metal complexes were further characterized by IR and UV-vis spectroscopy as well as magnetic susceptibility. In all cases, the magnetic moments were consistent with high spin complexes. We also completed a catalyst screening study intended to uncover correlations between metal atom type (Cr versus V), metal oxidation state (Cr(II) versus Cr(III)), and ligand structure on catalyst performance. Preliminary analysis of the polymerization data indicates that vanadium(III) complexes are invariably more active than their chromium(III) counterparts and that chromium(II) compounds are often (but not always) more productive catalysts than equivalent compounds prepared with chromium(III). Other than these general trends, we have yet to uncover a compelling correlation between catalyst performance and ligand structure, thus we have postponed publication of our results until a clearer picture emerges. The synthesis, characterization and polymerization testing was completed by an undergraduate co-worker, Daniel Bates. The work provided Dan with experience in all aspects of organic and inorganic compound synthesis and purification, characterization, and polymerization testing. He used aerobic and anaerobic synthesis techniques and applied various spectroscopic tools (IR, UV-vis, and NMR), along with mass spectrometry and magnetic susceptibility measurements, to completely characterize the organic and inorganic products. Dan also completed a research report summarizing his results. These experiences have prepared Dan well for his graduate career at During the past year we also continued our research collaboration with Chevron Phillips Chemical Company (CPChem). The CPChem work utilized a unique donor-modified a-diimine ligand in combination with metal halides, MCl2 (M = Mn, Fe, Co). The metal compounds (3) were prepared via an in-situ synthesis technique (Scheme 3) in which the tridentate ligand was formed in the presence of the transition metal salt. For nitrogen and phosphorus containing pendant donors, the tridentate ligand coordinated in a mer fashion, forming high spin complexes with square pyramidal or trigonal bipyramidal structures. For thioether pendant donors, a variety of structures were observed – ranging from monomeric tridentate (sulfur-bound), to monomeric bidentate (solvent bound), to dimeric with bridging chloride ligands – depending on the substitution pattern of the thioether and the solvent choice. A complete account of the synthesis and characterization of these compounds was published in a recent Dalton Transactions article. Polymerization testing showed that sulfur and phosphorus containing complexes were especially active catalysts, producing high purity a-olefins (up to 99.9 % alpha content) with activities over 230,000 g oligomer/g catalyst per hour for the diphenylphosphino-based pendant donor. Somewhat surprisingly, compounds comprising nitrogen-containing pendant donors displayed poor activity, suggesting that soft pendant donors (S, P) are preferable. Further details of catalyst activity and oligomer product properties were published recently in Organometallics. In addition to the two publications mentioned above, the CPChem work resulted in the submission of three patent applications to the US Patent Office (with one patent issued [1]), and a Council on Undergraduate Research (CUR) article [2] describing the CPChem/UW-Eau Claire research collaboration. The Organometallics and Dalton Transactions papers gratefully acknowledged PRF support. We intend to extend the pendant donor a-diimine ligands to chromium and other early transition metals over the coming months. _____________________________________________________________________________________ [1] US Patent 7,129,304 "Diimine Metal Complexes, Method of Synthesis, and Methods of Using in Oligomerization and Polymerization" (2006) [2] "Polymerization Catalyst Development: Collaborative Research with Chevron Phillips Chemical Company" M. J. Carney CUR Quarterly, September 2006, Vol. 27, No. 1, 22-26.