
ACS PRF | ACS
All e-Annual Reports

45283-AC10
Porous and Biphasic Materials through Solid State Reactions
We are investigating routes to porous and functional inorganic materials and bifunctional composites via solid-solid and solid-vapor reactions. By utilizing spontaneous, template-free processes, we are developing versatile, rapid routes which are broadly applicable, as evidenced by the wide variety of materials systems already investigated. These materials may be utilized in many fields, including filtration, catalysis, magnetic data storage, charge trapping, etc.
Reactive dip coating
Utilizing local interdiffusion of transition metal species on the surface of porous oxides has permitted the formation of nanoscale ternary oxide precipitates on a polycrystalline matrix. After dipping porous NiO in a solution of lanthanum acetate, firing at moderate temperatures results in a coating of La2O3 particles on the pore walls. Subsequent firing at higher temperatures fully converts the sub-micron coating particles into La4Ni3O10. This diffusion-driven process leads to precipitates which are much stabler and well-adhering than conventional nanoparticles deposited on inert supports for catalytic applications.
Vapor-phase leaching
In the quest to produce porous materials via volume loss, pore-opening must occur at temperatures where nano-sized pores will remain open. Heating oxides in a flowing stream of 5% H2/N2 provides a reducing environment that allows conversion between transition metal oxides and metals. Reduction of Mn3O4 to MnO occurs at about 450°C, and is accompanied by significant volume loss. This volume loss is manifested in an open network of 20-100 nm pores. Because the oxygen sublattice is conserved during the process, extensive reconstruction of the grains does not occur. The crystalline orientation is therefore maintained, producing pores with aligned, square edges over the extent of each grain.
After reduction of macroporous Mn3O4 to MnO, we have closed the induced micropore network by reoxidation to Mn2O3. The macropore network is maintained, and subsequent reduction of Mn2O3 back to MnO reopens the micropores. This is a truly novel display of morphological regeneration in an oxide.
By applying hydrogen reduction to mixed-metal ternary oxides, we have selectively reduced individual components. Solid-state reduction of the inverse spinel Zn2TiO4 at 800°C leads to evaporation of Zn from the structure while the TiO2 is retained:
When both components remain in the structure, as in the case of NiMn2O4, we demonstrate the facile conversion of a single-phase material into an intimately mixed, highly-interfacial composite. At 725°C, the the reduction reaction proceeds in two distinct steps as:
Thin film applications
The precision and ordering of the porosity in vapor-phase leached materials is especially apparent in our single-crystalline MnO thin films. ZnMn2O4 was deposited hydrothermally on (100) and (111) MgAl2O4 substrates. Reduction of these epitaxial films in 5% H2/N2 at 450-650°C removes Zn and O, resulting in MnO films shown in Fig. 2a with an open network of micropores. On (100) substrates, all pore faces are aligned exactly parallel or perpendicular to the substrate. On (111), the pore edges are rotated by 54.75° with respect to the substrate (Fig. 2b). Pore sizes are 50-100 nm on (100), and 30-50 nm on (111). The epitaxial agreement between MgAl2O4 and ZnMn2O4 allows for tailoring of the pore orientation and size simply by choosing the plane of growth. Because the reduction occurs at temperatures much less than the melting point of MnO (1840°C), pore channels remain fully open during firing, and the microstructure does not depend on film thickness.
Personal Impact
Since receiving support from the PRF, Ram Seshadri has received tenure in the UCSB Materials Department. Eric S. Toberer has earned his PhD in Materials. He is currently in a postdoctoral position at Caltech and was awarded a Beckman Fellowship. Graduate student Daniel P. Shoemaker has been researching functional biphasic materials since Fall 2006.
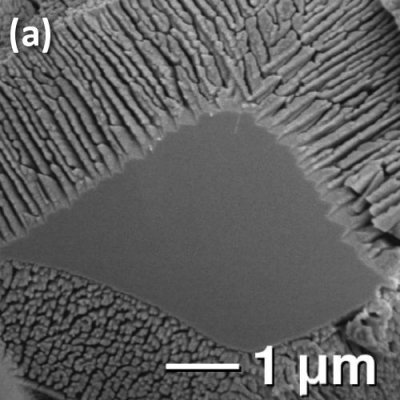
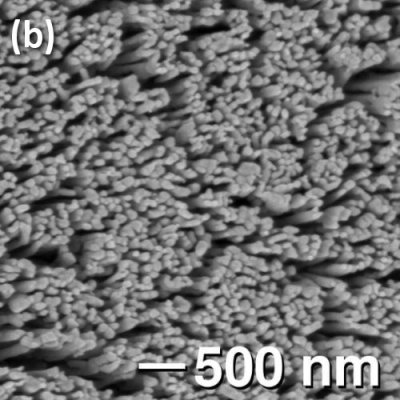 Fig. 1. (a) Intermediate reduction in 5% H2/N2 of Zn2TiO4 results in TiO2 dendrites supported on an unreduced core. (b) Complete reduction leads to arrays of TiO2 nanorods.
Fig. 1. (a) Intermediate reduction in 5% H2/N2 of Zn2TiO4 results in TiO2 dendrites supported on an unreduced core. (b) Complete reduction leads to arrays of TiO2 nanorods.
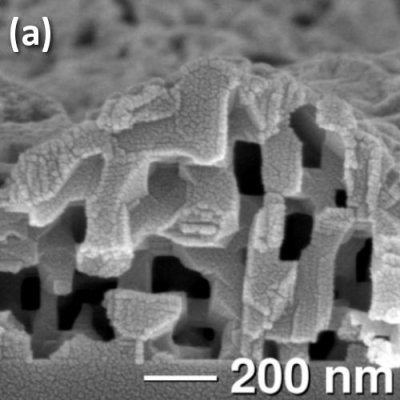
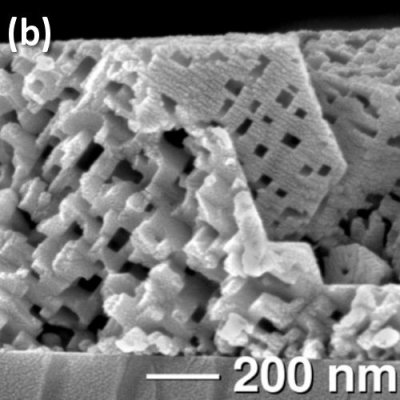 Fig. 2. Epitaxial ZnMn2O4 films on MgAl2O4 are reduced to MnO, resulting in open, aligned porosity while preserving crystallinity. Films grown on (a) the (100) plane and (b) (111) plane of the substrate display different pore sizes and orientations with respect to the growth plane.
Fig. 2. Epitaxial ZnMn2O4 films on MgAl2O4 are reduced to MnO, resulting in open, aligned porosity while preserving crystallinity. Films grown on (a) the (100) plane and (b) (111) plane of the substrate display different pore sizes and orientations with respect to the growth plane.