
ACS PRF | ACS
All e-Annual Reports

43890-B5
Gemini Surfactant Clay Intercalates for Triphase Catalysis
We continued working on triphase catalysis (TC) as a unique type of phase transfer catalysis (PTC) in which the catalyst and each of a pair of reactants are located in different phases (Fig. 1). In the case of TC, the third phase is generally a solid phase such as a synthetic polymer or clay mineral. The reactants from the two immiscible liquid phases (usually water and an organic solvent such as toluene) are transferred to the solid interface where they react. In a typical nucleophilic displacement reaction, which we have carried out under triphase conditions, the organic phase contains the substrate (e.g. n-butyl bromide), and the nucleophilic reagent (e.g. Cl -) is in the aqueous phase as shown in Fig. 1. Hectorite from the smectite clay family has been used to support quaternary onium cations; in particular we have used novel divalent Gemini surfactants as catalysts (solid phase). TC shows considerable potential for commercial application due to the ease of catalyst removal from the reaction mixture.
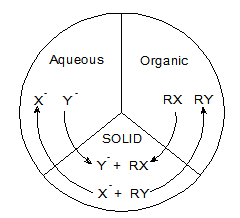
Figure 1. Schematic representation of a triphase catalytic system. The reactants from each liquid phase come to the surface of the solid phase (clay) and react.
In the last year and a half, our group has examined several hectorite clay intercalates as catalysts using Gemini surfactants in order to study the kinetics of a nucleophilic displacement reaction converting n-butyl bromide to n-butyl chloride.
n-butBr (org) + Cl - (aq) - - - > Gemini Clay Intercalates - - - > n-butCl (org) + Br - (aq)
Quaternary onium cations consist of a single “hydrophilic head” connected to a “hydrophobic tail”. In contrast, Gemini (or dimeric) surfactants consist of two conventional single-tail surfactants whose heads are connected by a “spacer” chain (Fig. 2).
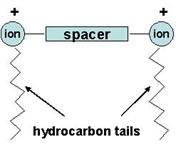
Figure 2. General schematic representation of a Gemini surfactant.
These surfactants have shown quite unusual phase behaviors and have recently motivated numerous studies. Because of their hydrophilic heads and hydrophobic tails, surfactant molecules form “self assemblies” or supra-molecular aggregates such as micelles, vesicles, etc. in aqueous solvent. Considering the structural characteristics of the Gemini amphiphile, which is constructed with two hydrophobic chains connected by one or two hydrophilic head groups, molecular design and tailoring in Gemini surfactants are of great interest to us to investigate the behavior and catalytic properties of Gemini surfactant clay intercalates.
We have synthesized and characterized several Gemini surfactants with various spacer size and hydrocarbon tails. Surfactants with spacers of hydrocarbon chains of 2 to 6 carbon atoms and hydrocarbon tails from 2 to 8 carbon atoms were synthesized and successfully intercalated using hectorite clay. Our preliminary results have indicated that clay intercalates with Gemini surfactant catalyst with spacer groups longer than 4 carbon atoms are comparable or better in catalytic activity than traditional quaternary ammonium catalyst.
Our major focus up to this point has been devoted to the synthesis and characterization of various Gemini surfactants and their intercalation chemistry. In the next phase of this research effort, we are planning to put more emphasis on the comparative kinetics of the nucleophilic displacement reaction using various traditional quaternary onium salts and Gemini surfactant intercalates.
This interdisciplinary area of research at SIUE has had a great impact on the careers of my undergraduate students. Erica Beverlin worked on the Gemini synthesis and intercalation chemistry. She presented a poster at the 233rd ACS National meeting, Chicago, IL March 2007, and she is now in medical school working toward an M.D. at St. Louis University. Danielle Reed worked in reaction kinetics using triphase catalysts, and she is now working toward an M.S. degree under my supervision. Two new undergraduate students, Timi Parker and Neha Parikh, have recently joined my research group. We have submitted a paper from student work to Tetrahedron Letters in August 2007.