
ACS PRF | ACS
All e-Annual Reports

42285-GB4
Dynamics of DNA Hybridization
Background
Stable single stranded oligonucleotide conformations, such as hairpins, play an important role in biological functions such as gene expression since they have the capability of acting as a binding site for proteins. Hairpins consisting of tetraloops (see Figure 1) are abundant in genomes and therefore, there have been several reports on their structure. This has sparked interest in studying the thermodynamic and kinetic characteristics of hairpins. In a previous report, the hybridization rates for sequences containing stable tetraloops with varying numbers of complements in the stem were presented. In addition, DNA analogs (LNA's) were used in various regions of the hairpin to study the effects on the thermodynamics and kinetics of hybridization to the complementary strand. LNAs are composed of a 2'O, 4'-C-methylene bridge in the sugar. They can hybridize to DNA strands and form a duplex with a high degree of stacking, similar in structure to A-DNA where the DNA sugars are in both S-type and N-type conformations. The stacking in these duplexes results in a structure that is more thermodynamically stable and duplexes where some of the DNA has been replaced with LNA (LNA + DNA) or where DNA has hybridized to a complementary LNA strand (DNA:LNA) typically have increased melting temperatures compared to the all-DNA hybrids.
Hybridization kinetics of duplexes formed from tetraloops with LNA substitutions in the loop showed that the rates of hybridization were higher compared to the all-DNA structures. LNA substitutions in the stem of the hairpin decreased the rates of hybridization compared to the all-DNA duplexes and substitutions in the end regions had little to no effect on the rates. To further understand the role of LNA in the structure of hairpins, thermodynamic studies were performed on single strands. Results
Melting curves of hairpins containing a GAAA tetraloop with four and five complementary bases in the stem were obtained using UV-Vis spectroscopy. Similar experiments were performed with sequences containing LNA substitutions in the loop. A van't Hoff analysis was performed from the melting curves and the melting enthalpies, entropies, and Gibbs free energies were calculated.
Figure 2 shows a single stranded melting curve of a GAAA tetraloop obtained from a sequence with four complementary pairs in the stem (like that shown in Figure 1). The upper and lower arms of the melting curve were fitted to a straight line. The fits were used to find the Tm, the temperature where half of the DNA was in the duplexed state, and the equilibrium constant, K, for the melting process, assuming a two-state model. Figure 3 shows a van't Hoff plot for the four-base tetraloop. Similar analyses were performed on melting curves of tetraloops with five complementary bases in the stem and tetraloops with LNA substitutions in the loop. Using the van't Hoff equation,
![]()
the entropy and enthalpy change associated with melting was obtained. Table 1 summarizes the data obtained from this study.
The results in Table 1 show that LNA substitutions into the loop of the hairpin decreased both the melting enthalpy and entropy of the hairpin, compared to the all-DNA structure. In previous studies, the increase in stability of the duplexes containing LNA (LNA+DNA:DNA) was attributed to either a decrease in the entropy associated with duplex formation or an increase in base stacking in the duplex. In this study, it is proposed that the hairpins containing LNA in the loop have limited degrees of freedom compared to the all-DNA structures because of the restriction of the sugar pucker. This would give rise to a lower entropy of melting for hairpins containing LNA in the loop (observed in Table 1). However, in the hairpin structures containing LNA in the loop, the enthalpy of melting is also lower, compared to the all-DNA hairpins. The lower enthalpy is probably due to a decrease in base stacking and a change in the interactions between the bases in the loop. Overall, the Gibbs free energy of melting for DNA hairpins correspond to a more stable structure compared to the values for hairpins with LNA in the loop.
Figures

Figure 1: Schematic of a GAAA tetraloop showing
four-base complementarity in the stem
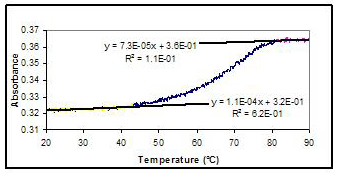
Figure 2: Melting curve for a GAAA hairpin with four
complementary bases in the stem. The linear fits to the
lower and upper arms are shown.

Figure 3: A van't Hoff plot used to obtain the entropy
and enthalpy change associated with melting.

Table 1: thermodynamic values obtained from the melting curves. Tloop4 and
Tloop5 are all-DNA hairpins, L4Tloops have LNA substitutions in the loop and
L4sTloop has LNA substitutions in the stem. The code for sequences is:
upper case = DNA; lower case = LNA; Bold = bases in the loop