
ACS PRF | ACS
All e-Annual Reports

43304-AC7
Phosphorylide Mediated Polymerizations of Acrylic Monomers
Addition of triphenylmethylpotassium in THF to tetraphenylphosphonium (TPP) bromide produces the corresponding triphenylmethide (TPP, TPM) that can form ylides by addition of the TPM anion or ester enolates to the 4-position of one of the phenyls (Zagala, A.P.; Hogen-Esch, T.E. Macromolecules 1996, 29, 3038; Baskaran, D.; MŸller, A. H. E.; Kolshorn, H.; Zagala, A. P.; Hogen-Esch, T. E. Macromolecules 1997, 30, 6695). These ylides rapidly initiate MMA and give living polymerizations with PMMA polydispersities (PDI's) comparable to nucleophilic group transfer (GTP) polymerizations (Webster, O. W.; Hertler, W. R.; Sogah, D. Y.; Farnham, W. B.; RajanBabu, T. V. J. Am. Chem. Soc. 1983, 105, 5706; Webster, O. W. Adv. Polym. Sci. 2004, 167, 1-34.)
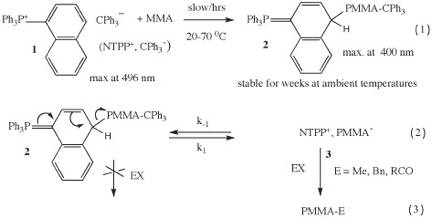
Scheme-1. Formation of PMMA ylides by addition of MMA anion to NTPP ion.
For the case of 1-naphthyltriphenylphosphonium triphenylmethyl salt, 1 (NTPP,TPM) TPM addition occurs exclusively at the 4-position of the 1-naphthyl group giving the corresponding TPM ylide that rapidly initiates MMA to give the corresponding PMMA ylide, 2. (Scheme-1, eqn. 1). Both ylides are stabilized by recapture of the full aromaticity of the remaining naphthalene moiety (See: Dimov, D.K.; Warner, W.N.; Hogen-Esch, T.E Macromol. Chem. Phys., Macromol. Symposia. 2000, 157, 171).
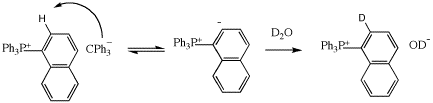
Scheme-2. Deprotonation of ortho proton of NTPP cation reaction by TPM anion.
Ylide, 2, gives far slower MMA polymerizations than the TPP ylide (factor of about 10,000) but gives excellent MW control (PDI<1.10) and at much higher temperatures (at least 70 0C). Polymerization kinetics and DFT calculations (Weiss, H.; Steiger, S.; Jungling, S.; Yakimansky, A.V.; MŸller, A.H.E. Macromolecules 2003, 36, 3374) have indicated that the polymerizations occur exclusively through the PMMA anion or ion pair. However, apparent initiator efficiencies for the polymerizations mediated by both ylides were consistently below 100 percent.
PRF support has allowed us to show that:
(A) Formation of 1 and the analogous TPP ion pair, at ambient temperatures, is accompanied by deprotonation by the TPM anion of the protons ortho to phosphorous (Scheme-2. See also: J. Ling and T. E. Hogen-Esch Macromolecules 2006, 39, 9665). These reactions are prevented by generating the TPM,NTPP (or TPP,TPM) salts at -30 °C followed by addition of a few equivalents of MMA. Warming to ambient temperatures gives the corresponding ylides that show quantitative MMA initiation efficiencies at ambient conditions. However, ylide, 2, is unusually stable at ambient (weeks or longer) or higher temperatures and give even better MW and PDI control than the TPP ylides.
(B) End-functionalization of PMMA anion with alkyl or benzyl halides (EX) is not trivial due to competing Claisen reactions of the PMMA enolate. Hence PMMA alkylations are typically carried out for 1 day or longer at -78 0C. However NMR shows that the reaction of 2 with benzyl bromide and related alkyl halides in THF at ambient temperatures is quantitative in about 30 minutes making this ylide a convenient way, at least in principle, to end-functionalize PMMA (J. Ling and T. E. Hogen-Esch Macromolecules 2007, 40, 5706). The reason for this is the controlled release of extremely small fractions (~ 10-6) of highly reactive anionic PMMA, 3, in the presence of a large concentration of EX that allows rapid PMMA anion alkylation compared with its spontaneous termination. The coupling of PMMA anion with one half molar equivalent of bis-1,4-(bromomethyl) benzene was also found to be quantitative and reactions with acyl halides likewise appear to be well controlled (J. Ling and T. E. Hogen-Esch unpublished). Thus PMMA functionalizations of this type are now achievable and this should allow the synthesis of new branched PMMA architectures i.e. star-, mikto-arm star or cyclic PMMA. The kinetics of the above alkylation and acylation reactions at ambient temperatures were also carried out and provided semiquantitative data on the rate constants in the absence of small counterions that are known to affect rates. Given the low concentrations of the PMMA, NTPP ion pair (~10-9-10-10 M) it is plausible that the ionization into free PMMA anions is substantial and perhaps largely complete. Finally, the unusual stability of naphthyl ylides may allow applications in the synthesis of ester enolates derivatives.
PRF funding has allowed the extensive training of a postdoctoral associate (J. Ling) in anionic polymerization and characterization techniques. The grant has also allowed training of postdoctoral and graduate students in vacuum line work. The results of this work were published in two articles in Macromolecules (see above) and were presented at the September 2006 ACS meeting in San Francisco and at the September 2007 biannual IUPAC meeting on "Ionic Polymerizations" in Bayreuth, Germany. The PRF support has also allowed us to purchase chromatographic and related equipment.