
ACS PRF | ACS
All e-Annual Reports

40690-AC7
Surface Transitions at Comb Polymer Interfaces Using Synchrotron X-ray and Infrared-Visible Sum Frequency Generation Spectroscopy
 Poly(n-alkyl acrylate)s (Fig. a) exhibit surface freezing, whereby a single ordered monolayer is formed at the surface of the isotropic liquid bulk above the bulk melting temperature (Tm).1,2 The surface freezing temperature range above Tm (DT) in poly(n-alkyl acrylate)s is significantly higher compared to their small molecule analogs, i.e. alkanes.3 The range of DT increases to 7-20 K from 1-3 K by attaching n-alkanes to a polymeric backbone using acrylate linkage.
Poly(n-alkyl acrylate)s (Fig. a) exhibit surface freezing, whereby a single ordered monolayer is formed at the surface of the isotropic liquid bulk above the bulk melting temperature (Tm).1,2 The surface freezing temperature range above Tm (DT) in poly(n-alkyl acrylate)s is significantly higher compared to their small molecule analogs, i.e. alkanes.3 The range of DT increases to 7-20 K from 1-3 K by attaching n-alkanes to a polymeric backbone using acrylate linkage.
We have performed grazing incidence X-ray diffraction (GIXD) measurements at X-ray synchrotron source, Argonne National Laboratory to understand the difference in DT between poly(n-alkyl acrylate)s and alkanes. Our 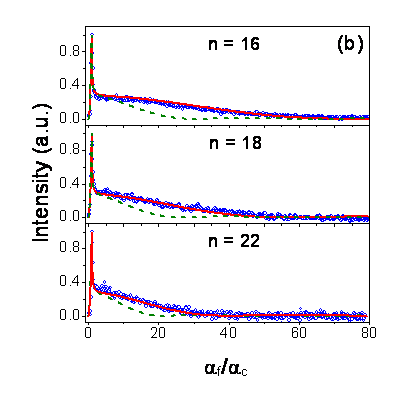 results suggest that only a fraction of the side chain length is crystalline in the surface frozen layer. A theoretical calculation based on GIXD results successfully explains the difference in DT between poly(n-alkyl acrylate)s and alkanes.
results suggest that only a fraction of the side chain length is crystalline in the surface frozen layer. A theoretical calculation based on GIXD results successfully explains the difference in DT between poly(n-alkyl acrylate)s and alkanes.
Grazing Incidence X-Ray Diffraction Measurements: Fig. b shows the intensity along the Bragg rod for poly(n-alkyl acrylate)s (n = 16, 18, 22) in GIXD measurements as a function of diffraction angle (af) normalized by the critical angle of polymer-air interface (ac). The data were obtained in the surface frozen phase. Good fits to the experimental data are obtained only for the partial crystallinity of the surface side chains (solid lines). The trends assuming the complete length of the surface side chains to be crystalline are shown as dotted lines. Our fits suggest that the number of alkyl units in a surface side chain not taking part in crystallization is constant (» 9) for poly(n-alkyl acrylates). Partial crystallinity of the surface side chains in poly(n-alkyl acrylate)s is also supported by the lower entropy 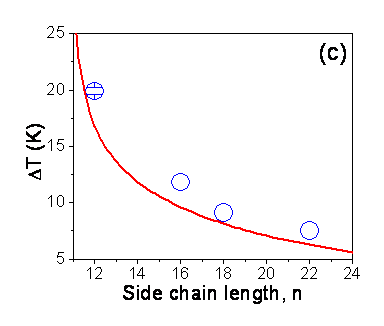 change on surface melting by our surface tension measurements.4
change on surface melting by our surface tension measurements.4
Theoretical Calculation of DT: Figure (c) shows DT for poly(n-alkyl acrylate)s as a function of side chain length obtained from the previous surface tension and SFG measurements.1,4 Melting temperature is given as the ratio of energy change to entropy change upon transition. Hence, surfaces melt after bulk in case surface melting is accompanied by larger energy change or lower entropy change in comparison to the bulk counterpart. In case of long alkyl chain containing systems, well-packed methyl surface groups in the crystalline phase has lower energy compared to the melt surface. Extra thickness of the surface frozen layer in poly(n-alkyl acrylate)s compared to n-alkanes due to the involvement of linker group leads to higher energy change on surface melting for polymers.2 In addition, partial crystallinity of surface side chains lowers the entropy change on surface melting for poly(n-alkyl acrylate)s. The calculation of DT taking these two factors into account is shown in Fig. (c) as a solid line. Good agreement exists between the experimental and theoretical values of DT.
In conclusion, we have studied the surface frozen layer in poly(n-alkyl acrylate)s using grazing incidence X-ray diffraction. Our results suggest partial crystallinity of the surface side chain. The theoretical calculation based on GIXD results explains the magnitude of DT in these polymers. The surfaces of poly(n-alkyl acrylate)s can be tweaked by surface frozen layer without affecting the bulk properties,1,5 important for their application as smart adhesives, adhesive tapes, seed coatings, personal care products, and food packaging films. Our study provides better understanding of this unique surface effect and will lead to experimental work aimed towards the search of linker groups resulting in still higher DT.
References
1. Gautam, K. S.; Dhinojwala, A. “Melting at Alkyl Side Chain Comb Polymer Interfaces” Phys. Rev. Lett. 2002, 88, 145501/1-4.
2. Gautam, K. S.; Kumar, S.; Wermille, D.; Robinson, D.; Dhinojwala, A. “Observation of Novel Smectic –Like Phase Above the Bulk Melting Temperature” Phys. Rev. Lett. 2003, 90, 215501/1-4.
3. Ocko, B. M.; Wu, X. Z.; Sirota, E. B.; Sinha, S. K.; Gang, O.; Deutsch, M. “Surface Freezing in Chain Molecules: Normal Alkanes“ Phys. Rev. E 1997, 55, 3164-3182.
4. Prasad, S.; Hanne, L.; Dhinojwala, A. “Thermodynamic Study of a Novel Surface Ordered Phase above the Bulk Melting Temperature in Alkyl-Side Chain Acrylate Polymers” Macromolecules 2005, 38, 2541-2543.
5. Prasad, S.; Hanne, L.; Dhinojwala, A. “Surface Segregation in Polymer Blends Driven by Surface Freezing” Macromolecules 2006, 39, 7467-7470.