ACS PRF | ACS
All e-Annual Reports

44979-G5
Studying Monolayer Formation on Alloys
In this reporting period we have made progress on investigating the metal oxide-organic interaction on stainless steel 316L and its component oxides by working on the two goals of the proposal: 1. forming organic monolayers on the surface and varying the organic head group pKa from acidic to basic to determine if acidity of the head group is the controlling factor in reactivity; 2. studying the surface metal role in monolayer formation and stability by using the alloy and its individual components.
Goal 1: Prior to this grant we had successfully formed monolayers on the oxide surface of SS316L using octadecylcarboxylic acid and octadecylphosphonic acid. The conditions for monolayer formation developed by our laboratory were mild solution deposition conditions at low concentrations (1mM) and atmospheric pressure followed by several hours in a warm (120°C) oven. We proposed to expand this study to form monolayers, using mild conditions, with molecules whose pKas range from 4 to 15. In this reporting period we have attempted monolayer formation with amines, alcohols, sulfonic acids and alkanes. All attempted surface modifications were analyzed by diffuse reflectance infrared spectroscopy. If modification was successful, the substrates were rinsed in the deposition solvent and sonicated to test the chemical and mechanical stability of the monolayer. Films stable to these tests were then analyzed by AFM and MALDI-TOF MS.
Octadecylamine formed ordered films upon deposition with the νCH2 asymm = 2916 cm-1. But after rinsing in THF for 10 minutes, most of the monolayer was removed and the amine left on the surface was disordered with νCH2 asymm centered at 2925 cm-1.Thus we can conclude that though amine SAMs may be formed due to the electrostatic interaction of the charged NH3+ head group with the oxide surface of stainless steel 316L, they are not strongly bound to the surface. The deposition of sulfonic acids has led to inconsistent data. In some cases, surface modification leads to strongly bound multilayers while other trials lead to no surface modification. The details of the experimental procedure are being worked out at this time. Despite using higher heat, lower pressure, and increasing concentration monolayers were not formed on the oxide surface of SS316L by either octadecanol or octadecane. This result is not surprising because octadecanol has a high pKa and has not previously been reactive towards oxide species. The reactivity of the organics to SS316L substrate is, thus far, found to follow the order: phosphonic acid > carboxylic acid > amine > alcohol > alkane. This is consistent with increasing reactivity with increasing pKa.
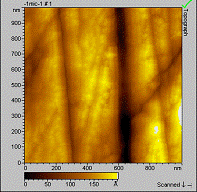
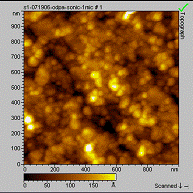
Goal 2: The surface of SS316L is composed of oxides of the following metals: Fe 66.01%, Cr 19.19 %, Ni 9.17%, Mn 3.22% and Mo 2.42%. To determine the role of the surface components in monolayer formation we used each metal oxide as a substrate for monolayer formation. The results are summarized in Table 1. Only octadecylcarboxylic acid and octadecylphosphonic acid have been thoroughly tested on these surfaces. Both of these molecules form well-ordered, stable monolayers on SS316L. Monolayer formation with octadecylphosphonic acid was straightforward on all of the substrates, while carboxylic acid only formed monolayers on iron and molybdenum oxides. Interestingly, these unreactive surface species do not prohibit formation of a complete monolayer of carboxylic acidson SS316L. The monolayers have been characterized by infrared spectroscopy and atomic force microscopy (Figure 1). Contact angle measurements and MALDI-TOF MS analysis are planned for the next reporting period.
Metal Oxide
| Octadecylphosphonic acid
| Octadecylcarboxylic acid
|
Iron
| Ordered
| Ordered
|
Chromium
| Ordered
| No monolayer formation
|
Nickel
| Ordered
| No monolayer formation
|
Manganese
| Ordered
| No monolayer formation
|
Molybdenum
| Ordered
| Ordered
|
Table 1. Type of monolayers formed on metal oxide substrates. Ordered= An ordered monolayer with complete coverage and stable to rinsing.
Figure 1. AFM image of the nickel oxide surface before (left) and after (right) deposition of octadecylphosphonic acid. The rms roughness before and after deposition was 38Å, indicating the formation of a uniform film on the surface. In the upcoming year, the project will include the use of different organic molecules in monolayer formation, including hydroxamic acid and sulfonic acid, on both the alloy and metal oxides. All substrates will be analyzed using MALDI-TOF MS to determine film height, contact angle goniometry, infrared spectroscopy and AFM.