ACS PRF | ACS
All e-Annual Reports

42853-AC1
Asymmetric Synthesis via Organocopper Chemistry
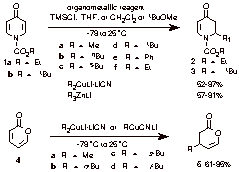
The aim of this project is to develop efficient asymmetric synthesis strategies involving organocopper chemistry employing either enantioenriched cuprates or enantioenriched electrophiles (e.g., propargyl and allylic substrates) or both at the same time. We have found that 4-pyridones 1a-1b undergo 1,4-conjugate addition reactions with orgaonozinc and cuprate reagents to afford 5,6-dihydropyridin-4-ones 2-3 in good to excellent yields, setting the foundations for the examination of chiral ligands and the development of asymmetric variations of this transformation. Cuprate mediated conjugate addition reactions to 2-pyrone (4) to afford dihydropyrones 5a-d have been developed despite literature suggestions that cuprates fail to add to pyrones in useful yields. With these developmental studies in hand, efforts will be directed toward examination of asymmetric cuprate conjugate additions mediated by chiral external ligands.
Work has also been directed toward controlling the chemo-, regio-, and stereoselectivity in the reactions of organocuprates with gamma,delta-heteroatom substituted alpha,beta-enoates. A one pot organocopper mediated tandem allylic substitution on epoxyenoate 6 afforded the 2,3-disubstituted-4-enoate 7 with good chemo- and regioselectivity, but gave poor diastereoselectivity. Utilization of sequential allylic substitution reactions on ethyl 4-halo-5-acetoxy-2,3-hexenoate gave excellent chemo-, regio-, and stereoselectivity to afford initially 8 and then 9a in 85% yield with a dr = 93:7. The selectivities in these reactions were sensitive to the cuprate reagent, the solvent, and to some small degree the temperature. These developments suggest that excellent sterocontrol can be achieved in these sequential allylic substitution and efforts are underway to broaden this strategy for the synthesis of O and N-heterocycles. Support for this project has enabled us to develop preliminary results that provide proof of concept for utilizing tandem allylic organocopper substitutions for generating contiguous stereogenic centers in a stereocontrolled fashion. We anticipate extending this work to the use of tandem sequential allylic substitutions and 1,4-additions mediated by both palladium and copper reagents. This support by ACS-PRF has enabled one student to nearly complete his Ph.D. work and another to make substantial progress toward a degree.