ACS PRF | ACS
All e-Annual Reports

45215-AC10
Synthesis and Polymorphic Control for Visible Light Active TiO2 Nanoparticles
Synthesis and Polymorphic Control for Visible Light Active TiO2 Nanoparticles
1.0 Research Activities
During the first year, research was focused on synthesis of titania nanoparticles and characterization including the photocatalytic activities.
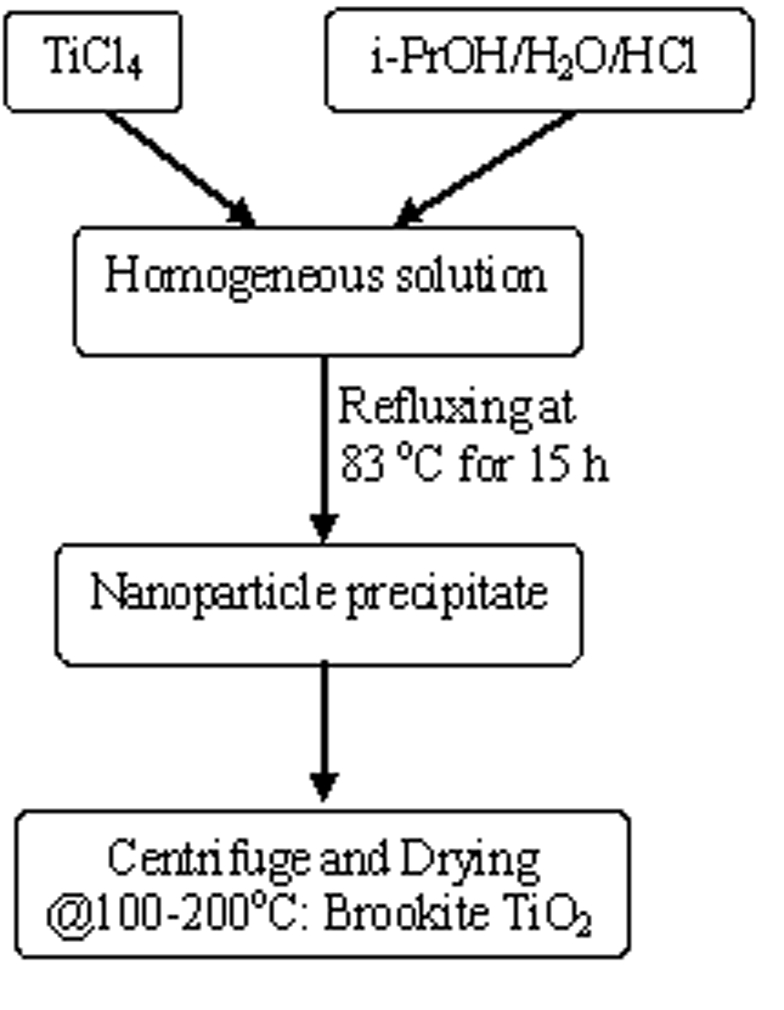
Brookite TiO2 nanoparticles were prepared by WACS
process as shown in Figure 1 as described in our previous papers [1-4]. The
SACS technique (U.S. Patent appl. pending*) developed by our
research group [5] has been used as a post-synthesis treatment of WACS process.
Reaction specifics can be seen in Table 1. All calcined TiO2
powders were heat-treated in air at 200oC for 2 hours.
Figure 1. Brookite TiO2 synthesis diagram
Table
1. Experimental Conditions utilized to produce single phase and mixed phase
titania
Sample ID Major TiO2 Phase Mode of Formation Temperature (°C) Solvent HCl concentration(M) Time (hr) WACS Brookite WACS 83 2:1 IPA:water 0.3 15 WACS-200 Brookite Calcination of WACS 200 - - 2 SACS* Brookite SACS * * - * SACS-200 Brookite Calcination of SACS 200 - - P25 Anatase Obtained from Degussa N/A - - 2 *U.S. Patent applied & info withheld. 1.1.2 Characterizations All samples were characterized by N2
Adsorption and X-Ray Diffraction (XRD). The photocatalytic activities
were evaluated by the degradation of the methyl orange (MO) under UV
irradiation. The characterization and photocatalytic
activity test conditions were reported in the publications from our group
[2-4].
1.2
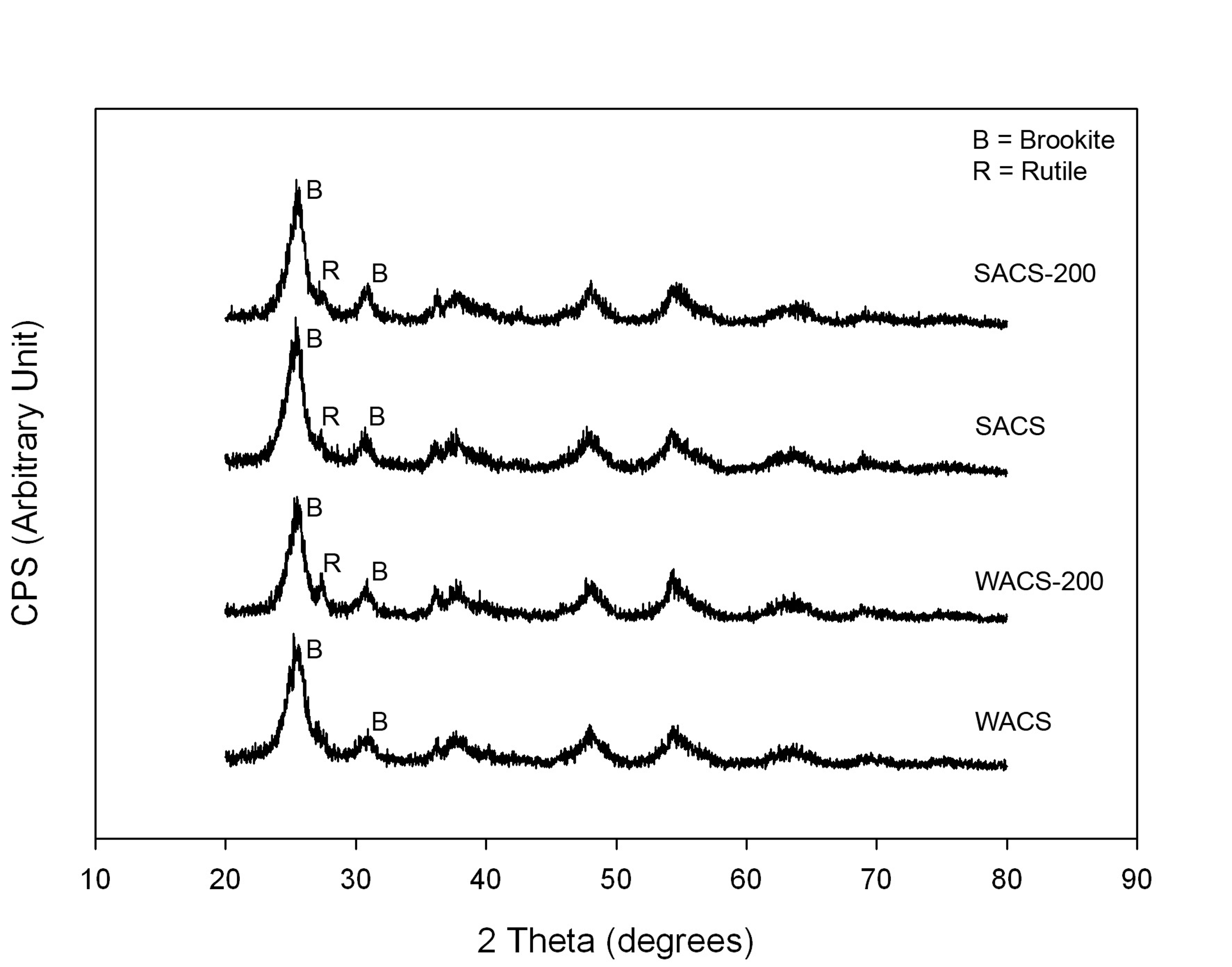
Results The X-ray diffraction patterns of the TiO2
particles are shown in Figure 2. The TiO2 phase of uncalcined WACS
and SACS samples is predominantly brookite. Calcined titania samples at 200oC
for 2 hours had a small portion of rutile in the structure. The crystallite
sizes calculated from the XRD data as reported in Table 2 are approximately 16
nm which is much smaller than the P25 crystalline size.
The results of BET surface area, pore volume, and average
pore diameter of titania samples are given in Table 2. The BET surface area of
our brookite titania samples were three times larger than that of P25 samples.
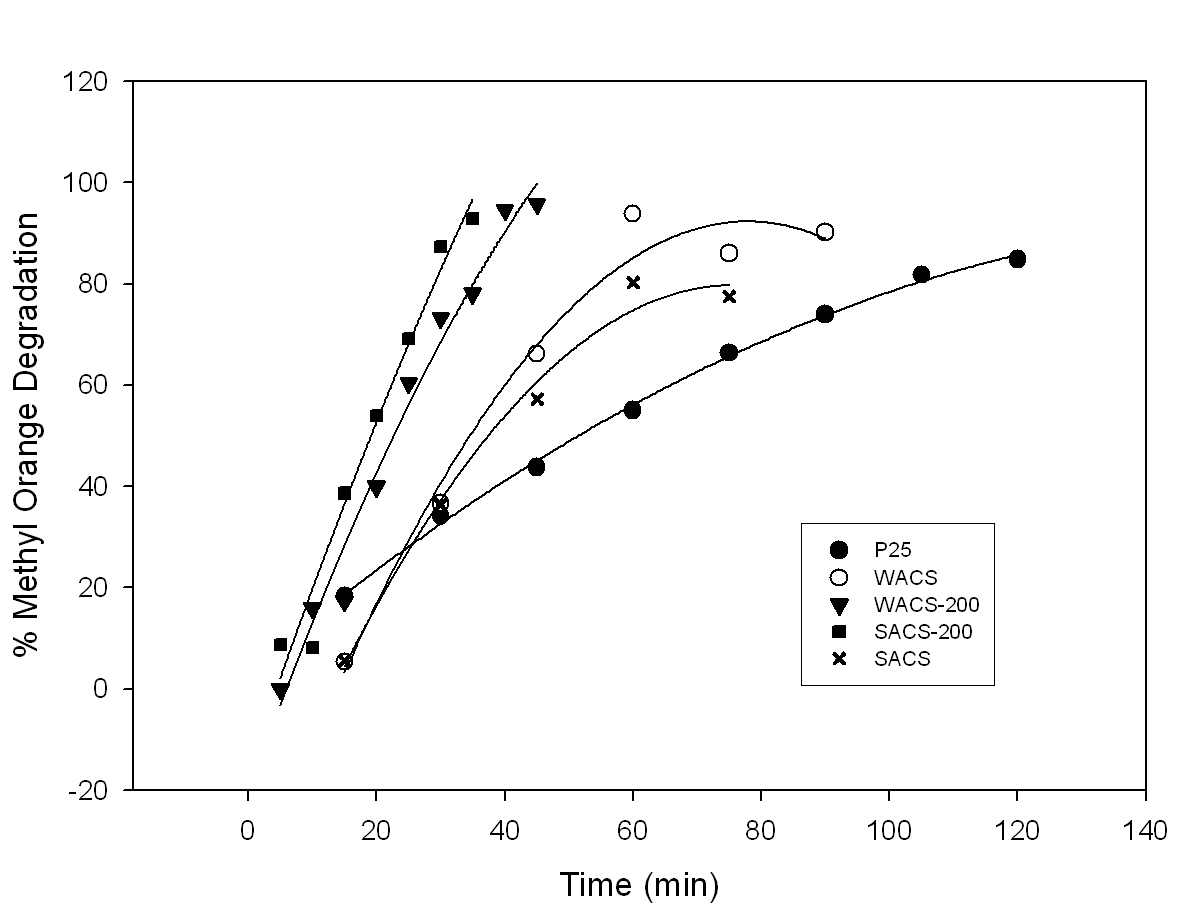
The photocatalytic activities of brookite titania samples compared to P25
samples, determined by the methyl orange degradation, are shown in Figure 3.
The degradation by the uncalcined samples is significantly slower than those
samples calcined at 200°C for 2 hours, due to the removal of surface hydroxyls
and/or organic. The photocatalytic activities, obtained by MO degradation, of
the calcined brookite titania samples, WACS-200 and SACS-200, are much better
than the commercial P25 sample. A few papers from our group [2,4] reported
that our brookite titania is more photocatalytically active than P25. The
orange color of MO for SACS-200 was degraded ten minutes faster than that of
WACS-200 (*further info withheld). This is supported by data obtained from
FTIR and 1H NMR analysis and the measured pore volume of samples. The
SACS brookite titania paper was submitted to catalysis letters journal in
August 2007.
The antibacterial properties were
obtained from collaboration with Biological Sciences Department, Clemson University. UV light activated brookite titania on Escherichia coli 23848 killed
all bacteria within 120 minutes, but P25 did not [6].
Figure 2. The XRD pattern of the brookite titania
samples
Table 2. The physical properties of prepared titania
samples and the reference P25.
Sample ID Crystalline size (nm)* BET surface area (m²/g) Pore Volume (cm³/g) Pore size average (Å) Brookite Anatase Rutile WACS 14 - - 163 0.1 25 WACS-200 18 - - 157 0.1 27 SACS 12 - - 197 0.1 28 SACS-200 13 - - 202 0.2 30 P25 - 21 40 56 0.2 169 * calculated from XRD data using the Scherrer equation
Figure3. Methyl orange
degradation of brookite titania samples compared to commercial P25.
2.0
Conclusions Up to now, we can conclude that brookite titania is
photocatalytically and antimicrobially superior to P25. Solvothermal treatment
technique enhanced the photocatalytic properties of brookite titania. We found
that our brookite titania has potential to be active under visible light. The
future work will focus more on doped titania to improve visible light
photoactivation.
3.0 References [1] B.I. Lee, X. Wang, R.
Bhave and M. Hu, Mater. Lett. 60 (2006) 1179.
[2] C.A. Nolph, D.E.
Sievers, S. Kaewgun, C.J. Kucera, D.H. McKinney, J.P. Rienties, J.L. White, R.
Bhave and B.I. Lee, Catal. lett. 117 (2007) 102.
[3] R.C. Bhave and B.I.
Lee, Mater. Sci. Eng. A 467 (2007) 146.
[4] R.C. Bhave, C.A. Nolph, S. Kaewgun and B.I. Lee, Catal. Comm. (2007).
[5] L. Qi, B.I. Lee, P.
Badhekaa, L. Wang, P. Gilmourc, W.D. Samuels and G.J. Exarhos, Mater. Lett. 59
(2005) 2794.
[6] R.R. Shah, Masters
Dissertation, Department of Biological Sciences, Clemson University (2007).