ACS PRF | ACS
All e-Annual Reports

44891-B3
Preparation, Characterization, Film Fabrication, and Photoluminescence of Metal-Organic Networks of Copper(I) Cyanide
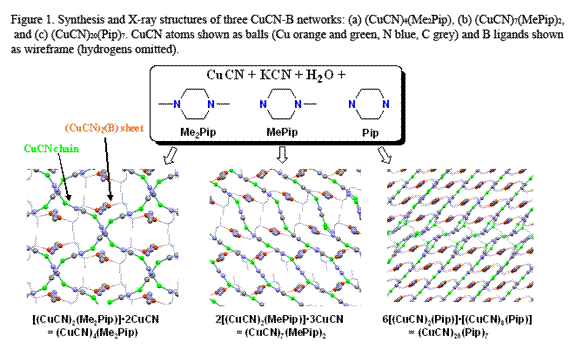
Copper(I) cyanide networks have been formed with diimine ligands (B) including 4,4'-bipyridyl (Bpy), pyrazine (Pyz), quinoxaline (Qox), phenazine (Phz), pyrimidine (Pym), quinazoline (Qnz), pyridazine (Pdz), and phthalazine (Ptz) and with diamine and tetramine ligands (B) including DABCO, hexamethylenetetramine (HMTA) and piperazines (including N-methyl, N-ethyl, and N-phenyl substituted piperazines). Stable, highly luminescent (CuCN)x(B)y networks self-assembled in single-step, high-yield, aqueous reflux reactions of CuCN and B in the presence of KCN.
Over twenty new crystal structures have been solved, revealing CuCN:B = 1:1, 3:2, 2:1, 5:2, 3:1, 7:2, and 4:1. In many cases multiple phases are encountered for a given B ligand. The variety of copper coordination found includes 2-, 3- and 4-coordination and both m2-cyano (Cu–C≡N–Cu) and m3-cyano (Cu–C(–Cu)≡N–Cu) bridging. In the latter case the two copper atoms bridged by the cyano carbon atom are usually bonded (Cu…Cu distance <2.8 Å). A common network motif is the (CuCN)2B (6,3) hexagonal network, which consists of hexagonally tiled Cu6(CN)4B2 units as a result of bridging B and m2-CN groups. There is no metal-metal bonding in the (6,3) network.
The more copper-rich phases incorporate 2-coordinate Cu centers, often as independent (CuCN)∞ chains which are threaded through (6,3) networks. Thus, for the two networks shown in Figure 1a and 1b “(CuCN)4(Me2Pip)” is actually [(CuCN)2(Me2Pip)]•2CuCN and “(CuCN)7(MePip)2” is actually 2[(CuCN)2(MePip)]•3CuCN (Me2Pip = N,N'-dimethylpiperazine, MePip = N-methylpiperazine, Pip = piperazine). A unique and highly emissive network, (CuCN)20(Pip)7 is shown in Figure 1c. The actually formulation of (CuCN)20(Pip)7 is 6[(CuCN)2(Pip)]•[(CuCN)8(Pip)]. In this case the CuCN chains that thread through the (CuCN)2(Pip) sheets are themselves linked into loops forming a separate (CuCN)8(Pip) net. This is the first example of two non-identical, intepenetrated CuCN-L lattices within a single network structure. Twenty independent Cu centers and twenty site-disordered cyano groups are present. Borderline copper-copper interactions are present between the two networks (Cu…Cu = ca. 2.8-3.0 Å).
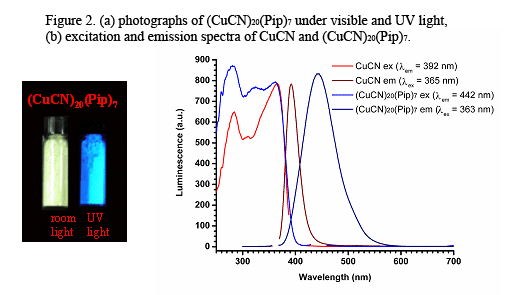
The new copper(I) cyanide networks show interesting spectroscopic behavior. CuCN itself is colorless, absorbing in the UV (see Figure 2a), but luminesces intensely at the border of the UV and visible. Most of the new diimine networks prepared are colored as a result of metal-to-ligand charge (MLCT) transfer. The new diamine and tetramine networks are more-or-less white. Several of the diimine complexes show weak intensity luminescence emission in the visible. This emission is probably stimulated by MLCT. The emission occurs in the yellow to red spectral region, but it is only seen for a few materials containing Bpy, Pym, Pdz and Ptz. Moreover, the emission is about 1000-fold weaker than that of CuCN, suggesting that diimine ligands have a luminescence quenching effect. On the other hand, the diamine and tetramine networks are often highly luminescent, in some cases slightly more so than CuCN itself. Emission is especially intense in Cu-rich phases, usually decreasing with decreasing copper content. Thus, emission intensity in the CuCN:Pip series decreases as follows: (CuCN)20(Pip)7 >> (CuCN)2(Pip) > (CuCN)3(Pip)2. The diamine and tetramine CuCN network emission is in the blue to yellow region. Visible and blacklight photographs of (CuCN)20(Pip)7 (as a representative luminescent network) are shown in Figure 2a. The excitation and emission spectra for CuCN and for (CuCN)20(Pip)7 are shown in Figure 2b. It is apparent that the excitation spectra of the two networks are similar. This suggests that similar excitation mechanisms are operating in both materials. Excitation is likely to be the result of Cu(I) d10 à d9s1 or d9p1 transitions. It will be noted that the (CuCN)20(Pip)7 emission is red-shifted by about 50 nm with respect to that of CuCN. It is also broadened. The red-shifting is thought to be the result vibrational relaxation afforded by spring-like action of B ligands. Emission broadening is believed to be the result of multiple copper environments found in (CuCN)20(Pip)7 and related materials.