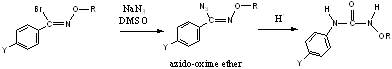ACS PRF | ACS
All e-Annual Reports

44412-GB1
New Synthetic Methods Using Oxime Ethers
The support from the PRF grant has had a profoundly positive impact on the career of the PI and the undergraduate students involved in this research. It has allowed for the successful completion of a study of nucleophilic substitution reactions of imidoyl halides by carbon nucleophiles. This work has not only given undergraduate students the chance to participate in novel research, it has resulted in a publication for the PI and her undergraduate researchers and in the opportunity to make presentations at national/regional meetings.
Under this grant the research group has investigated using N-alkoxyimidoyl halides as starting materials in the synthesis of oxime ethers of pure E or Z isomeric configuration, even in cases where there is little difference in the thermodynamic stability of the two isomers. As the oxime ether moiety in a single geometric configuration is important in some pharmaceutical and agricultural compounds, and as it is beginning to be used as a starting material in the synthesis of chiral amines,1 routes to forming a single geometric isomer of this functional group are important.
We have demonstrated that a single geometric isomer of an oxime ether can be formed from nucleophilic substitution of a (Z)-N-alkoxyimidoyl fluoride by a Grignard reagent.2 These reactions give moderate to good yields of an oxime ether with exclusively retained stereochemistry (Scheme 1). This synthetic route eliminates the need for separation of geometric isomers which is inherent in most other methods that introduce the oxime ether moiety.
Scheme 1: Nucleophilic Substitution of a (Z)-N-alkoxyimidoyl fluoride by a Grignard Reagent
We have also completed studies on nucleophilic substitution of N-alkoxyimidoyl fluorides by enolate-type ions. These substitutions result in novel carbon acids which display varying degrees of imine-enamine tautomerism (Scheme 2).2 This study adds to our understanding of the impact of electron-withdrawing groups and aromatic rings on the stability of the imine versus the enamine tautomer. The work outlined in Schemes 1 and 2 has resulted in a full paper, coauthored by two undergraduate researchers, which is currently in press in the Canadian Journal of Chemistry. Scheme 2: Nucleophilic substitution reactions of N-alkoxyimidoyl fluorides by enolate-type ions. R1 R2 R % Yield CO2Et CO2Et CH3 41% CO2Et CN CH3 56% CN CN CH3 46% CN CN i-Pr 62% Work has begun on substitution reactions of N-alkoxyimidoyl halides by the azide ion to form azido-oxime ether compounds. Preliminary studies of these new organoazides have found them to be surprisingly stable and sluggish to participate in typical reactions of organoazides (i.e., catalyzed pericyclic reactions). These compounds, however, have been found to undergo a Schmidt-type rearrangement in acidic media (Scheme 3). This rearrangement is currently being studied in detail and has resulted in collaboration with an industrial chemist and a computational chemist. Scheme 3: Synthesis of an azido-oxime ether and its Schmidt-type rearrangement under acidic conditions. The above work has resulted in numerous presentations at regional and national conferences. The PI has made three presentations at national ACS meetings and one presentation at an American Association of Pharmaceutical Scientists national meeting. Two undergraduate researchers have reported on this work at a Southwest Regional ACS meeting in References: 1. a) Enders, D.; Reinhold, U. Tetrahedron: Asymmetry 1997, 8, 1895-1946. b) Bloch, R. Chem. Rev. 1998, 98, 1407-1438. b) Moody, C. J. Chem. Commun. 2004, 1341-1351. c) Moody, C. J.; Gallagher, P. T.; Lightfoot, A. P.; Slawin, A. M. Z. J. Org. Chem. 1999, 64, 4419-4425. d) Dieter, R. K.; Datar, R. Can. J. Chem. 1993, 71, 814-23. e) Watson, K. G.; Brown, R. N.; Cameron, R.; Chalmers, D. K.; Hamilton, S.; Jin, B.; Krippner, G. Y.; Luttick, A.; McConnell, D. B.; Reece, P. A.; Ryan, J.; Stanislawski, P. C.; Tucker, S. P.; Wu, W.-Y.; Barnard, D. L.; Sidwell, R. W. J. Med. Chem. 2003, 46, 3181-3184. f) Palani, A.; Shapiro, S.; Josien, H.; Bara, T.; Clader, J. W.; Greenlee, W. J.; Cox, K.; Strizki, J. M.; Baroudy, B. M. J. Med. Chem. 2002, 45, 3143-3160. g) Rossello, A.; Bertini, S.; Lapucci, A.; Macchia, M.; Martinelli, A.; Rapposelli, S.; Herreros, E.; Macchia, B. J. Med. Chem. 2002, 45, 4903-4912. h) Ferrarini, P. L.; Mori, C.; Badawneh, M.; Calderone, V.; Greco, R.; Manera, C.; Martinelli, A.; Nieri, P.; Saccomanni, G. Eur. J. Med. Chem. 2000, 35, 815-826. i) Karakurt, A.; Dalkara, S. FABAD J. Pharm. Sci. 1999, 24, 143-156. 2. Dolliver,