ACS PRF | ACS
All e-Annual Reports

45567-AC1
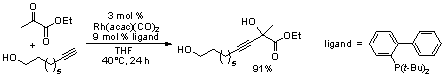
Rhodium-Catalyzed Addition of Alkynes to Activated Ketones and Aldehydes
Studies on the rhodium-catalyzed addition of alkynes to aldehydes and activated ketones are ongoing (eq. 1). During the past grant period experiments to help define the mechanism and attempts to find an asymmetric variant have been the primary focus. Numerous chiral phosphine ligands were used in an attempt to induce asymmetry to the process, but we have been unable to find a ligand system where high yield and enantioselectivity result using chiral phosphines. Attempts to modify the b-diketonate ligand will also be explored.
The expansion of transition-metal catalyzed nucleophilic addition reactions to the Henry reaction was also explored. Several literature reports describe metal complexes as excellent catalysts for the nitroaldol reaction. These transformations appeared to be easily modified into asymmetric processes, and therefore a reinvestigation of this work was undertaken. To facilitate ligand screening studies, attempts were made to generate the catalytically active metal complex in situ. A combination of [Rh(nbd)Cl]2 (nbd = norbornadiene) and tri-n-butyl phosphine provided the desired nitroaldol product in 46% yield. While this result appeared promising, use of tri-n-butyl phosphine alone gave a 91% yield, implicating the phosphine as the primary active catalyst. Further studies revealed that many electron rich phosphines were excellent catalysts for the Henry reaction (eq. 2). This study showed that care should be exercised in using metal-phosphine complexes as catalysts for the Henry reaction, as even small amounts of phosphine can lead to excellent yields of the nitroalcohol product.