
ACS PRF | ACS
All e-Annual Reports

44563-AC4
A Novel System for Production of 1,3-Propanediol Renewable Resources: Characterization of a B12-Independent Glycerol Dehydratase System
Progress Towards Aim 1. - Expression of the B12-Independent Glycerol Dehydratase System in A. vinelandii. Due to the oxygen sensitive nature of the catalytic [4Fe-4S]+1 cluster that is required for activity of the glycerol dehydratase activating enzyme (GD-AE) a reasonable hypothesis was to take advantage of an existing bacterium that has a demonstrated ability or “built-in” system for the protection of oxygen sensitive enzymes under aerobic growth conditions. Azotobacter vinelandii is one such organism3-5 that, in addition to the oxygen protection machinery, also has an established genetics system. The first step in achieving this goal is to demonstrate that active GD-AE can be expressed in A. vinelandii and active enzyme can be isolated.
Since the submission of this proposal we have looked at the recombinant expression of the GD-AE in A. vinelandii behind the nif promoter and the subsequent isolation and ability of the recombinant GD-AE to activate the glycerol dehydratase. These results are summarized in Table I.
GD-AE Source | Reconstituted? | GD Activitya on (S)-1,2-Propanediol | GD Activity on Glycerol |
E. coli (affinity purified) | Yes | 3.4 + 0.2 | 3.8 + 0.2 |
A. vinelandii (crude extract) | No | 0.6 + 0.2 | 0.8 + 0.2 |
A. vinelandii (affinity purified) | No | N.D.b | N.D. |
aSpecific activity reported in mmole·min-1·mg-1. bNo activity detected within the error of the experiment.
While, there appears to be a measurable activation of the GD with crude extract of the A. vinelandii, the relative yields for isolation of the GD-AE from either A. vinelandii or anaerobically grown E. coli have been discouraging (less than 5 mg from 200 g of cell paste). Whether this is due to degradation is not clear. Contradicting results have been reported for the role of the AdhE as an inactivator of the pyruvate formate lyase activating enzyme6,7, but it would seem likely that the cell would want to control radical generating enzymes at multiple levels. It may be possible to increase yields of the GD-AE by taking advantage of the DadhE knockout strain in E. coli.
Progress Towards Aim 2. – Biochemical, Spectroscopic, and Electronic Characterization of the GD-AE and the GD-(GD-AE) Complex. In order to take full advantage of radical SAM enzymes as industrial catalysts we are going to have to fully detail the mechanism by which these enzymes generate, stabilize, and transfer the radical species. Towards this end, we have identified a single point mutation in the GD that forms a stable complex with the GD-AE as well as a number of proposed mutations in the GD that will test our proposed mechanism for the dehydration of glycerol. Out of the seven mutations that were listed as a high priority in the initial proposal, two have been constructed and purified. These are the R782K variant and the H164N. In addition, we have also reconstructed all of expression plasmids and are using the 6x-poly His affinity tag instead of the StrepTag™ system. This was in large part due to the expense of the affinity resin associated with the latter system. We have further refined our coupled assay and upon initial submission of some work detailing the activation mechanism and dehydration of either 1,2-propanediol or glycerol by the GD, a reviewer pointed out that the kinetic constants for the DhaT enzyme we were using in the coupled assay (originally cloned from Clostridium butyricum) have not been reported. We have since investigated these kinetics (Figure 2). Given the amount of protein in the assay the specific activity is 36 mmol min-1 mg-1.
Observed Rate vs Calculated |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
0 |
20 |
40 |
60 |
80 |
100 |
120 |
Concentration 1,3-PD (mM) |
Velocity (microM/min) |
Observed |
Calculated |
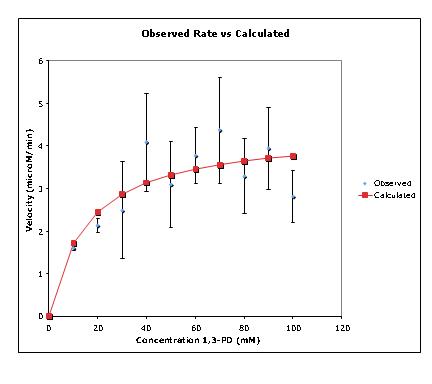
Figure 2. Kinetic data for the isolated DhaT enzyme.
A patent (WO0112833) has recently been reported that claims E. coli contains a non specific dehydratase (coded by the yqhD gene) that can perform the reduction of 3-HPA faster and to higher titers of 1,3-PD. Therefore, using this enzyme in place of DhaT may also be advantageous for our coupled assay of the B12independent glycerol dehydratase.
Future Work and Revised Strategic Plan.
Priorities still include i) completing the atomic model of R782K GD, ii) metal analysis for the GD-AE as isolated under both aerobic and anaerobic conditions, iii) completing an atomic model for the GD-AE alone or in a protein-protein complex with the R782K GD. While we can clearly reconstitute the activity of the GD-AE, it would be advantageous to be able to isolate substantially larger quantities of the active form of the enzyme directly. Therefore, we will also obtain or construct the DadhE E. coli strain for improved expression of either the GD-AE or the protein-protein complex between the GD-AE and the R782K GD. We will also clone, express, and isolate the gene product for the E. coli yqhD gene to use in the coupled assay.