
ACS PRF | ACS
All e-Annual Reports

43410-G3
Developing Late Metal Oxo Complexes of Strongly Donating Ligands
Insights into C-H activation by high valent iron imido complexes
A number of iron(IV) imido complexes [PhB(MesIm)3FeNR]OTf (R = tBu, Ad) are readily prepared by oxidation of the iron(III) precursors (Scheme 1). Despite their high oxidation state, these thermally stable complexes do not participate in nitrene transfer reactions with substrates such as alkenes and phosphines. However, they are able to activate reasonably strong C-H bonds, such as those in 1,4-cyclohexadiene (BDE = 77(1) kcal/mol).Scheme 1. Synthesis of [PhB(MesIm)3FeNR]OTf. Inset: X-ray crystal structure of [PhB(MesIm)3FeNAd]BPh4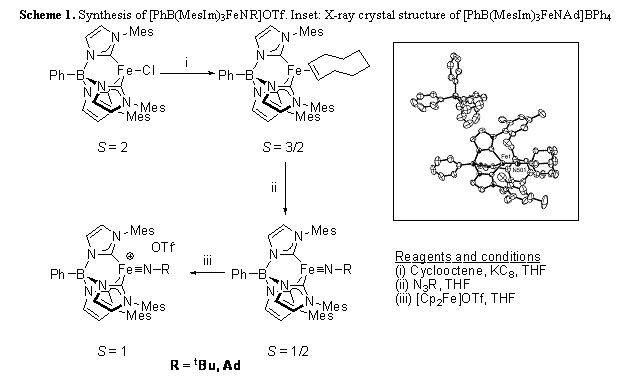
A thermodynamic investigation into hydrogen atom abstraction by these complexes was undertaken to provide insight into the experimental results (Scheme 2). Cyclic voltammetry of [PhB(MesIm)3FeNAd]OTf reveals a FeIII/IV couple at E1/2 = -0.98 V (vs Cp2Fe+/Cp2Fe), showing that despite the high oxidation state, these complexes are poor oxidants. The pKa of the product arising from hydrogen atom transfer, [PhB(MesIm)3Fe-N(H)Ad]+, was determined by a combination of experimental and computational methods, pKa(MeCN) = 37(3). These data allow the N-H BDFE (free energy) of [PhB(MesIm)3FeNAd]OTf to be calculated, N-H BDFE = 88(5) kcal/mol.
Scheme 2.

These results show that C-H activation reaction is driven by the high basicity of the iron(III) imido intermediate. The lack of reactivity towards group transfer may be due to the poor oxidizing ability of the iron(IV) imido complex, although steric effects cannot be eliminated at this stage. Therefore, these results provide insights into the driving forces for C-H activation as well as the factors that affect reaction selectively.
To provide insights into the effect of the metal oxidation state on C-H activation reaction, we have also begun to investigate the thermodynamics of hydrogen atom transfer to the iron(III) imido PhB(MesIm)3FeNtBu. It is notable that this complex can prepared by reaction of the stable 2,4,6-tri(tert-butyl)phenoxy radical (O-H BDE = 81 kcal/mol) with the iron(II) amido complex PhB(MesIm)3Fe-N(H)tBu. Thus, it is likely that the N-H BDFE of PhB(MesIm)3Fe-N(H)tBu is lower than of its iron(III) amido congener [PhB(MesIm)3Fe-N(H)tBu]+.
Electrophilic iron nitrido complexes
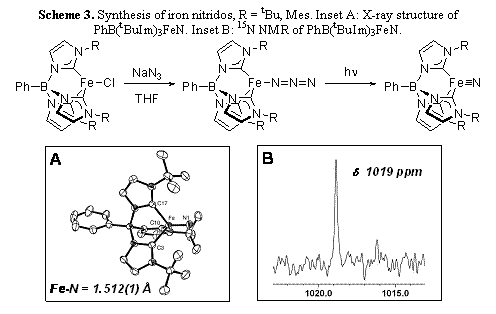
An exciting finding is that terminal iron nitrido complexes can be isolated and crystallized (Scheme 3). Photolysis of iron(II) azido complexes leads to the thermally stable iron nitrido complexes PhB(RIm)3FeN (R = tBu, Mes) in high yield. These are the first terminal iron nitrido complexes to be structurally characterized.
Initial reactivity studies show that the nitrido ligand has electrophilic character. Thus, PhB(tBuIm)3FeN reacts with PPh3 to form the imidophosphine complex PhB(tBuIm)3Fe-N=PPh3. Reaction of PhB(tBuIm)3FeN with electrophiles such as MeOTf, Me3SiOTf and Ph3COTf result in decomposition of the complex. A kinetic investigation of the reaction between PhB(tBuIm)3FeN and PPh3 gives the rate law rate = k[Fe][PPh3], with k = 3.65(6) × 10-4 M-1s-1 at 320 K. Preliminary results of a Hammett study shows that electron-donating substituents increase the rate of reaction (r =-0.66), consistent with nucleophilic attack of the phosphine at the nitrido ligand.
Scheme 3. Synthesis of iron nitridos, R = tBu, Mes. Inset A: X-ray structure of PhB(tBuIm)3FeN. Inset B: 15N NMR of PhB(tBuIm)3FeN.
We have begun investigations into N-H bond formation from the nitrido complexes. While acids have been found to decompose PhB(tBuIm)3FeN, the non-acidic hydride Co(dppe)2H, which possesses a very weak Co-H bond (Co-H BDE = 59 kcal/mol) reacts to form PhB(tBuIm)3Fe-NH2, with a selectivity of ca. 50 % (unoptimized). The parent amido complex PhB(tBuIm)3Fe-NH2 can be independently prepared from PhB(tBuIm)3FeCl and LiNH2. This suggests that hydrogen atom transfer may be an alternative method for creating N-H bonds.
While PhB(tBuIm)3Fe≡N does not react with H2, the more open complex PhB(MesIm)3Fe≡N reacts with 1 atm H2 at room temperature to form new diamagnetic product(s). While complete characterization of the product(s) from this reaction is still required, at this stage it is important to note that free ammonia is not detected.
4. Career Impact
The funding provided by the ACS-PRF has allowed the PI to conduct and disseminate research. The PI presented some of the results in this report as a poster at the Inorganic Reaction Mechanisms Gordon Conference in February 2007. This poster was one of eight selected for a 15 minute poster presentation. Results were also presented at the Organometallic Gordon Conference and the International Conference on Biological Inorganic Chemistry (ICBIC), both of which were held in July 2007. The PI was also selected to be a participant in the NSF-sponsored Inorganic Chemistry Workshop in June 2007. Three papers have been published from results obtained during the course of the award, two have been submitted, and another two are in preparation. The results in this report have been used as the basis for an NSF-CAREER proposal and an NIH-SCORE proposal. Finally, new spectroscopic collaborations have been established with Joshua Telser (