www.acsprf.org
Reports: UNI1050120-UNI10: Ionic Liquid Mobility in Electroactive Polymers
Jennifer A. Irvin , Texas State University (San Marcos)
BACKGROUND
Changes in redox states of electroactive polymers (EAPs) require movement of ions in order to maintain electroneutrality. Ion choice can affect morphology, electrochemical stability, and oxidation and reduction potentials. Ions are usually introduced as a solution of molecular electrolyte, although ionic liquids such 1-ethyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide (EMIBTI) have been investigated.[1-4]
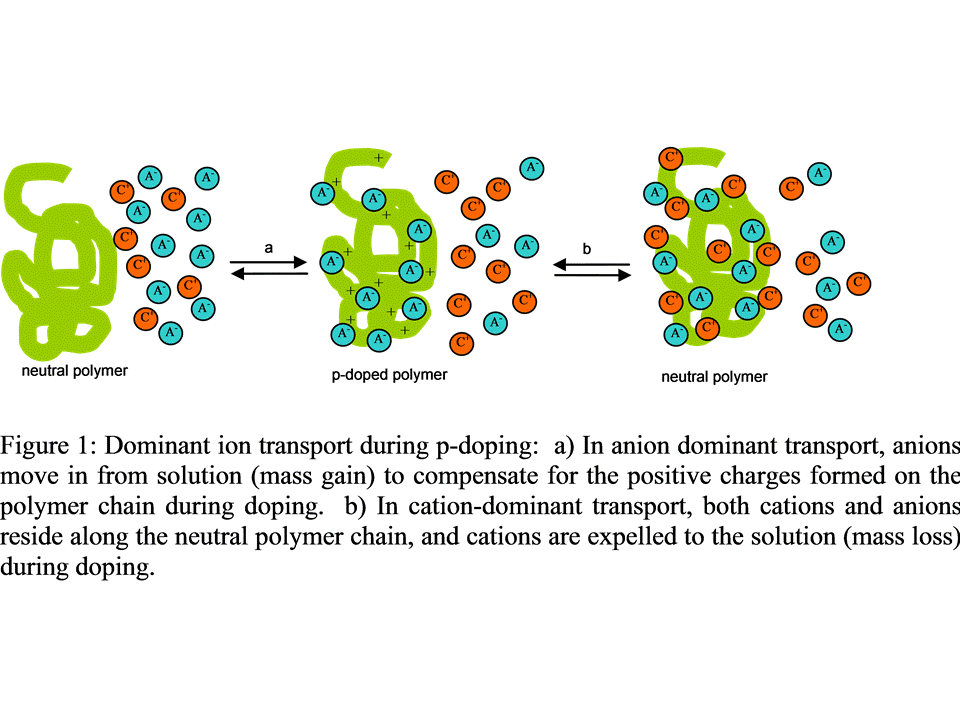
Electrochemical neutralization of p-doped polymers can be anion-dominant or cation-dominant (Figure 1).[5,6] Ion transport processes are best studied using an electrochemical quartz crystal microbalance (EQCM).[7,8] in which mass changes are monitored as a polymer is cycled between neutral and doped states.
While many EAPs are prepared as insoluble films via electrochemical oxidation of monomers, commercialization will most likely require solution-processable polymers.[9,10] Deposition of EAP films from solution often results in electrochemical performance inferior to that of similar electrochemically-deposited films.[11] While poor adhesion is often blamed for poor performance,[12] it is possible that dense polymer films may undergo oxidation/reduction processes only on the surface of the film.[13]
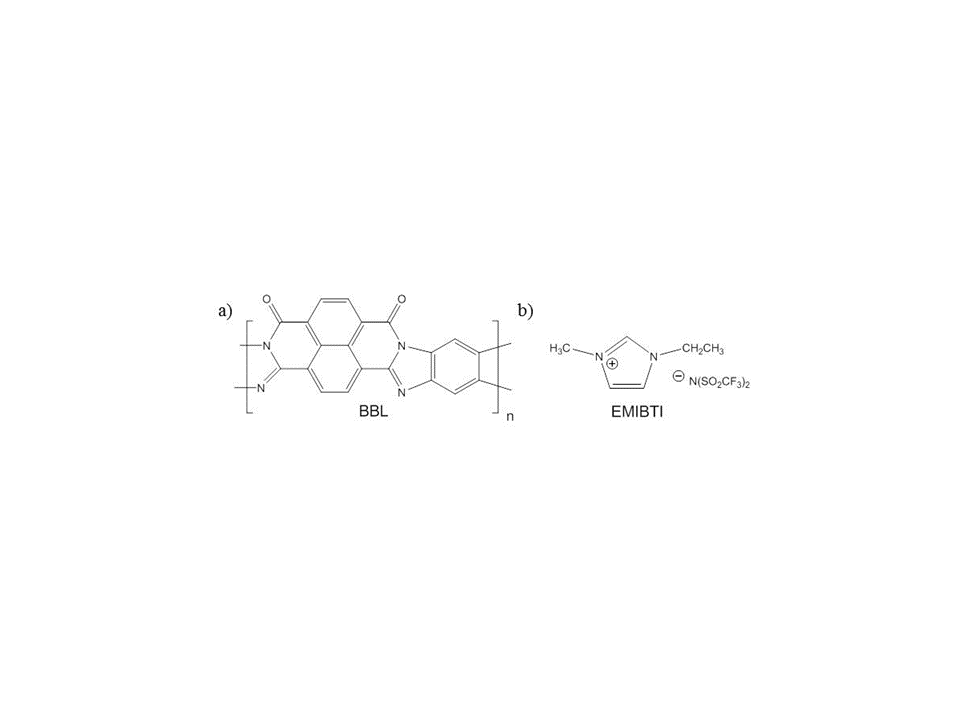
Here we report studies on films of the soluble n-doping ladder polymer, poly(benzimidazobenzophenanthroline) (BBL, Figure 2a) [14,15] co-cast with EMIBTI (Figure 2b) in hopes of improving polymer porosity and electroactivity.
Figure 2: Structures of a) poly(benzimidazobenzophenanthroline (BBL) and b) 1-ethyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide (EMIBTI)
RESULTS AND DISCUSSION
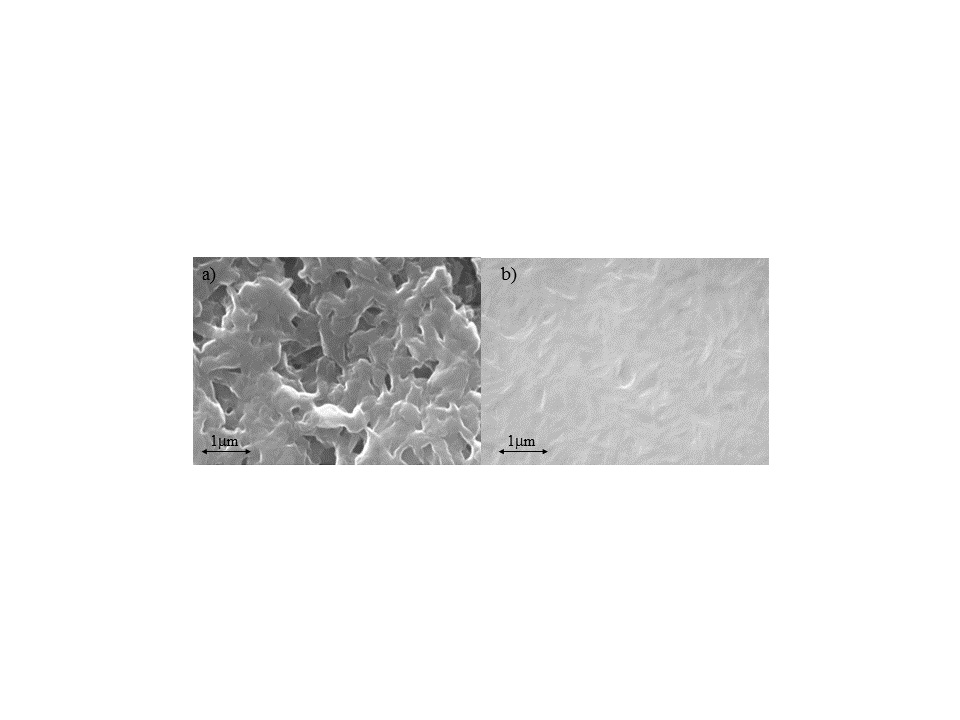
To prepare the solution, a concentration of 1 weight percent of a mixture containing 35 weight percent BBL and 65 weight percent EMIBTI was homogenized in MSA at 90°C under agitation for a period of ten hours. The ratio of BBL to ionic liquid was carefully selected based on volume fraction to yield a semi-continuous network of polymer and ionic liquid phases.[16] Scanning electron micrographs (SEMs) of BBL cast with (CC-BBL) and without (BBL) EMIBTI can be seen in Figure 3. It is apparent that CC-BBL has greatly increased surface roughness, which could indicate increased porosity.
Figure 3: Scanning electron micrographs of films of a) BBL cast with EMIBTI (CC-BBL) at 20,000x magnification; b) BBL cast without EMIBTI (BBL) at 20,000x magnification.
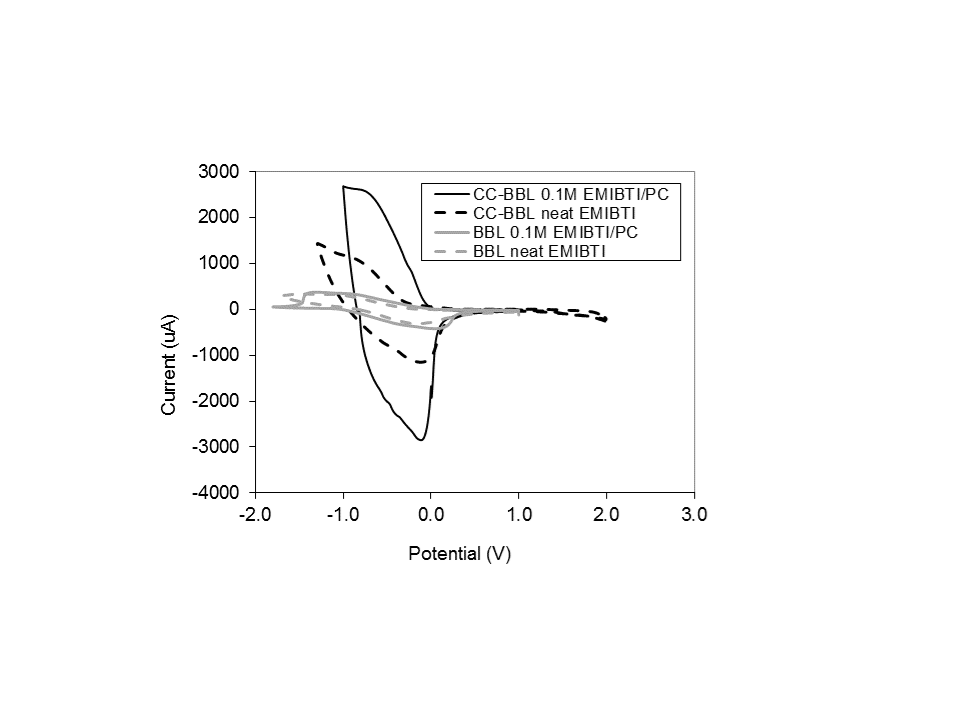
After rinsing to remove excess EMIBTI, the films were cycled one thousand times each in 0.1M EMIBTI/propylene carbonate, and the cyclic voltammograms (CVs) of the films were examined. CVs of both films in both 0.1M EMIBTI/propylene carbonate (solid lines) and neat EMIBTI (dashed lines) are presented in Figure 4. Current response is much higher in CC-BBL / 0.1M EMIBTI / propylene carbonate than in its BBL counterpart; this is likely a result of increased porosity in the CC-BBL film relative to BBL. Also note the impact of solvent in the electrolyte; current response for CC-BBL in neat EMIBTI is approximately half that in 0.1M EMIBTI/propylene carbonate. This is likely due to the relatively high viscosity of the neat EMIBTI resulting in electroosmotic drag, decreasing apparent electroactivity.[13]
Figure 4: Cyclic voltammetry of BBL cast with EMIBTI (CC-BBL, black) and without EMIBTI (BBL, gray); obtained by cycling BBL films in 0.1M EMIBTI/propylene carbonate (solid lines) or neat EMIBTI (dashed lines) vs. Ag wire at 50mV/s; the third cycle is shown for each polymer. Note that the same mass of BBL is used in all four films.
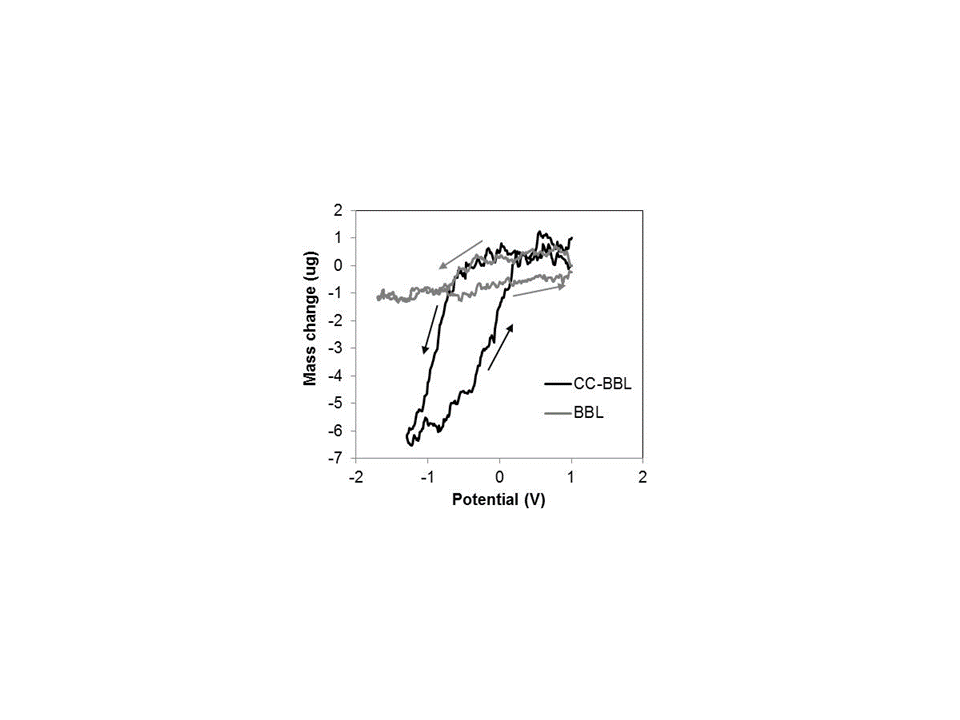
EQCM was used to monitor changes in mass as a function of applied potential. EQCM plots for the two BBL films in neat EMIBTI (corresponding to two dashed cyclic voltammograms presented in Figure 4) are shown in Figure 5. The mass change in the CC-BBL film is more than five times larger than the mass change that occurs in the BBL film; this is consistent with the observed increase in current response and is likely due to increased porosity in the CC-BBL film, resulting in a polymer that is more electroactive.
Figure 5: EQCM of BBL cast with EMIBTI (CC-BBL, black) and without EMIBTI (BBL, gray); obtained by cycling BBL films in neat EMIBTI vs. Ag wire at 10mV/s; the second cycle is shown for each polymer.
Both plots reveal a decrease in mass as the polymer films are reduced to the n-doped state. This is indicative of anions being excluded from the polymers as they are being reduced, i.e. an anion-dominant transport process.[17] In our EQCM studies, cations appear to remain in the polymer film throughout the n-doping process, much as large anions such as poly(styrene sulfonate) remain in a p-doping polymer throughout the redox process.[18]
CONCLUSIONS
We have produced thin films with a significantly improved initial activity as well as a high overall activity. The co-cast polymer morphology was found to be stable to over one thousand cycles without noticeable electrochemical degradation. This work was presented in an invited talk at the Southwest Regional Meeting of the American Chemical Society in Austin, Texas in November, 2011.
REFERENCES
[1] Pringle, J. M., et al., Polymer, 46, 2047, 2005.
[2] Stenger-Smith, J.D., et al., J. Electrochem. Soc., 149(8), A973, 2002.
[3] Stenger-Smith, J.D., et al., J. Electrochem. Soc., 157(3), A298, 2010.
[4] Stenger-Smith, J.D. and Irvin, J. A., Material Matters, 4(3), 103-105, 2010.
[5] Ding, J. et al., Chem. Mater., 15, 2392, 2003.
[6] Yang, H. and Kwak, J., J. Phys. Chem. B, 102, 1982, 1988.
[7] Niu, L., Kvarnstrom, C., and Ivaska, A., J. Electroanal. Chem., 569, 151, 2004.
[8] Krivan, E., Visy, C. and Kankare, J., Electrochim. Acta, 50, 1247, 2005.
[9] Song, S. et al., Talanta, 57, 263, 2002.
[10] D'Arcy, J. M. et al., Proc. Nat. Acad. Sci., 107, 19673, 2010.
[11] Crossland, E.J.W. et al., Adv. Funct. Mater., 21, 518, 2011, and references cited therein.
[12] Cui, X. and Martin, D.C., Sens. Act. A, 103, 384 2003.
[13] Pringle, J. M. et al., Polymer, 45, 1447, 2004.
[14] Arnold, F.E. and Van Deusen, R. L. Macromolecules, 2, 497, 1969.
[15] Arnold, F.E. , J. Poly. Sci. A: Polym. Chem., 8, 2079, 1970.
[16] Barlow, J. W. and Paul, D. R., Polym. Eng. Sci., 21, 985, 1981.
[17] Ferraris, J. P. et al., J. Electroanal. Chem., 459, 57. 1998.
[18] Plieth,W. et al., Electrochim. Acta, 51, 2366, 2006.