www.acsprf.org
Reports: UR150115-UR1: Expanding the Scope of the Diels-Alder Reaction: Development of Cationic Dienophiles Stabilized by Cobalt-Complexed Alkynes
Kevin M. Shea , Smith College
As described in my original proposal, the goal of this project is to develop a new class of cationic Diels-Alder dienophiles. Our guiding principle is to exploit the cationic stabilizing ability of cobalt-complexed alkynes, and our hypothesis is that cationic dienophiles incorporating cobalt will react faster and more efficiently with a variety of dienes. We planned to examine the reactivity of several cationic dienophiles and then explore their reactivity in tandem Diels-Alder/Pauson-Khand reactions for the synthesis of complex polycycles.
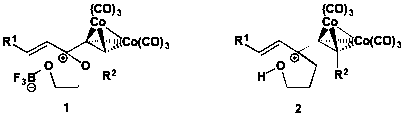
Our initial investigations focused on the development of two different classes of dienophiles: Gassman (1) and non-Gassman (2) types. Named for Paul Gassman, the pioneer in the study of cationic dienophiles, our Gassman-type dienophiles incorporate an oxygen atom that, along with the cobalt-complexed alkyne, stabilizes the reactive cation. In contrast, the non-Gassman-type dienophiles have a cation only stabilized by an adjacent cobalt-complexed alkyne. We focused our research efforts this year exclusively on the Gassman-type dienophiles.
Scheme 1. Examples of a Gassman- and a non-Gassman-type dienophile
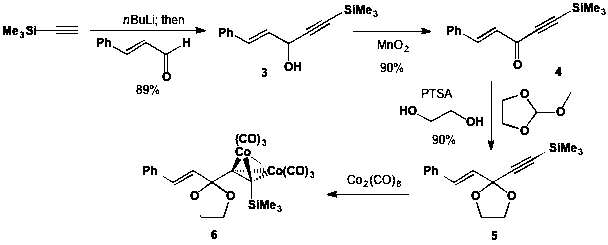
As reported previously, we have a robust synthesis of cobalt-containing dienophile precursor 6 as outlined in Scheme 2. Acetylide addition to cinnamaldehyde furnishes alcohol 3 (in 89% yield) which is subsequently oxidized in good yield to provide ketone 4. Ketalization with ethylene glycol yields ketal 5, and subsequent treatment with dicobalt octacarbonyl provides dienophile precursor 6.
Scheme 2. Synthesis of Gassman-type dienophile
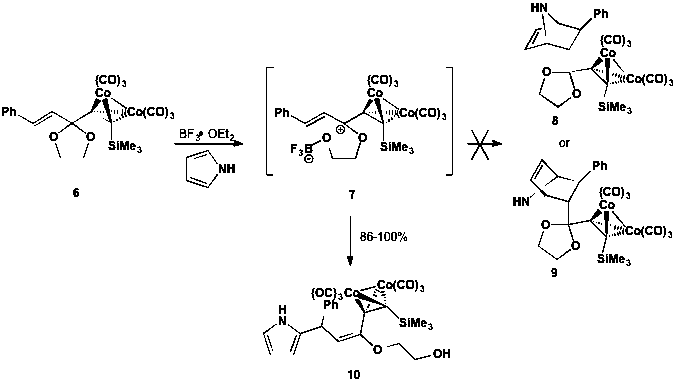
We previously reported that combination of dienophile precursor 6 with BF3¥OEt2 and pyrrole yields Diels-Alder product 8 or 9 (see Scheme 3). We reinvestigated this transformation and determined that, in fact, neither cycloaddition product 8 or 9 was formed. Instead, pyrrole participated in a vinylogous Nicholas reaction to yield substitution product 10 in high yield. This prompted further investigation into the reactivity of other aromatic dienes, and we confirmed that neither furan nor thiophene react with dienophile precursor 6.
Scheme 3. Vinylogous Nicholas reaction of a Gassman-type dienophile
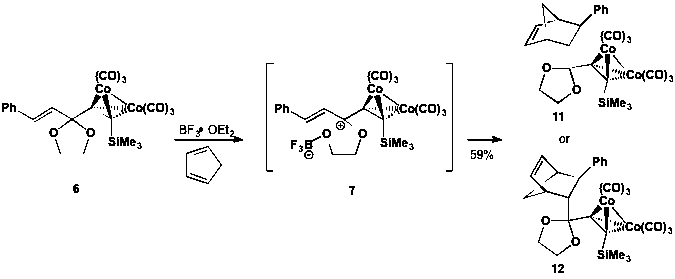
Our focus then shifted to reactions with carbocyclic dienes cyclopentadiene and 1,3-cyclohexadiene. We unambiguously determined that these reactive dienes do undergo Diels-Alder reactions with dienophile 7. As illustrated in Scheme 4, combination of 6 with BF3¥OEt2 and cyclopentadiene yields Diels-Alder product 11 or 12. We are still investigating the stereochemical outcome of the reaction via 2D NMR and derivitization then recrystallization and X-ray analysis to determine which diastereomer is formed. Optimization of this reaction demonstrated the importance of excess diene and addition of molecular sieves to suppress ketal deprotection by adventitious water. Thus, our optimized reaction furnished the target cycloadduct in 59% yield. Reaction of 1,3-cyclohexadiene in a similar manner yielded the corresponding Diels-Alder product, albeit in only 20% yield.
Scheme 4. Diels-Alder reaction of a Gassman-type dienophile with cyclopentadiene
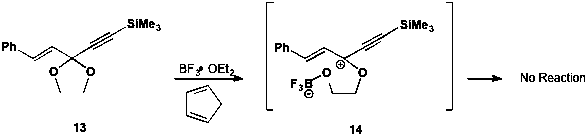
Our most exciting result was actually a Diels-Alder reaction that did not work. We demonstrated that reaction of 13, our standard dienophile precursor but lacking cobalt, under our optimized reaction conditions yielded only unreacted starting material. We observed this same lack of reactivity with 1,3-cyclohexadiene. Thus, we concluded that the presence of cobalt in 6 enables formation of the reactive dienophile 7, while 14 is unable to form under the same reaction conditions. This result validated our hypothesis and demonstrated the importance of the cobalt-complexed alkyne for generation of a cationic dienophile.
Scheme 5. Attempted Diels-Alder reaction without a cobalt-complexed alkyne
During the next year of our investigation, we will focus on reactions with acyclic dienes and unambiguously determine the stereochemistry of our Diels-Alder adducts.