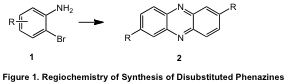www.acsprf.org
Reports: ND148955-ND1: Design and Synthesis of Novel Chiral Clefts and Helical Structures
Jeffrey Winkler, PhD , University of Pennsylvania
The ligation of aromatic rings represents an important tool for the synthesis of molecules of increasing complexity, with important applications in the synthesis of natural and unnatural products. We have developed strategy for the preparation of substituted phenazines leading to the synthesis of disubstituted heterocycles that cannot be otherwise prepared with comparable levels of efficiency (Figure 1).
Previous efforts by Beifuss and Senanayake have suggested that such a process should be possible, although with only single examples in unoptimized yields, both of which employ the highly pyrophoric ligand tri-t-butyl phosphine. During the last grant award period, we have optimized this process and developed it as a method of choice for the synthesis of unsymmetrically disubstituted phenazines. We have applied this aryl ligation reaction to tryptophan to generate the previously unreported heterocyclic ring system 1,7-dihydrodipyrrolo-[2,3-b:2',3'-i]phenazine, a highly fluorescent pentacyclic ring system.
Bromoanilines that are
substituted with either electron-donating or electron-withdrawing groups can be
regioselectively converted to the corresponding disubstituted
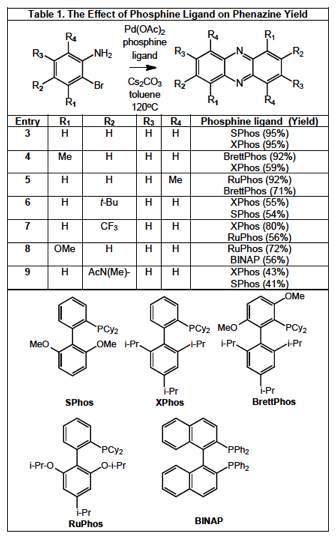
phenazines in good yields using BINAP or other crystalline phosphine ligands in lieu of tri-t-butyl phosphine (Table 1). Using the Buchwald C-N Ligand Kit available from Strem Chemicals, a series of phosphines were surveyed, and the two ligands that afforded the highest yields are presented in the Table for each substrate, illustrating the absence of a single optimal phosphine ligand in this reaction. Similar results were observed with the 2-chloroaniline (64% yield) although the corresponding
triflates resulted only in oxygen to nitrogen triflate migration in lieu of the desired coupling reaction. The last entry in Table 1 is particularly notable as, even though the observed yield is moderate, none of the desired phenazine product was observed with BINAP.
We have also examined the reaction of more complex aminoaromatics derived from amino acid precursors, as outlined in Figure 2. Aryl ligation of tryptophan using this strategy could lead to the previously undescribed dihydrodipyrrolophenazine ring system shown in 13. Toward that end, dimerization of 10, leads to the formation of the corresponding phenazine in 51% yield. However, all attempts to convert 11 to the ring-opened product under the reaction conditions described by Hino and coworkers led to none of the desired product 13. Direct dimerization of the N-Boc tryptophan 12 produced the desired ring system 15 in 49% yield. The UV spectrum of 13 was notable for a lmax of 406 nm, and the fluorescence spectrum of 13 featured a lem of 538 nm, with a Stokes' shift of 132.
 The
analogous reaction of the phenylalanine-derived substrate 14 afforded the corresponding phenazine 15 in 63% yield. A further example of the regiochemical control that is possible with this phenazine construction strategy is the formation of 17, albeit in 40% yield, from 16, without formation of the
corresponding dibenzo[a,j]phenazine,
a result that would not be possible using the more classical approaches to the
synthesis of substituted phenazines.
The
analogous reaction of the phenylalanine-derived substrate 14 afforded the corresponding phenazine 15 in 63% yield. A further example of the regiochemical control that is possible with this phenazine construction strategy is the formation of 17, albeit in 40% yield, from 16, without formation of the
corresponding dibenzo[a,j]phenazine,
a result that would not be possible using the more classical approaches to the
synthesis of substituted phenazines.
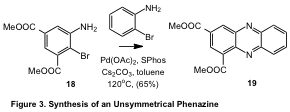
Finally, we have demonstrated that this strategy is not limited to dimerization. By judicious choice of substituents on the aniline ring, i.e., by exploiting the decreased reactivity of anilines substituted with electron-withdrawing groups, we have demonstrated that the reaction of 18 with 2-bromoaniline leads to the formation of 19 in 65% yield. The application of this methodology to the synthesis of diverse substituted phenazines is currently underway in our laboratory and our results will be reported in due course.